The Development and Characterization of a High Efficiency Pyrimidine Derivatives Corrosion Inhibitor for Acid Jobs
-
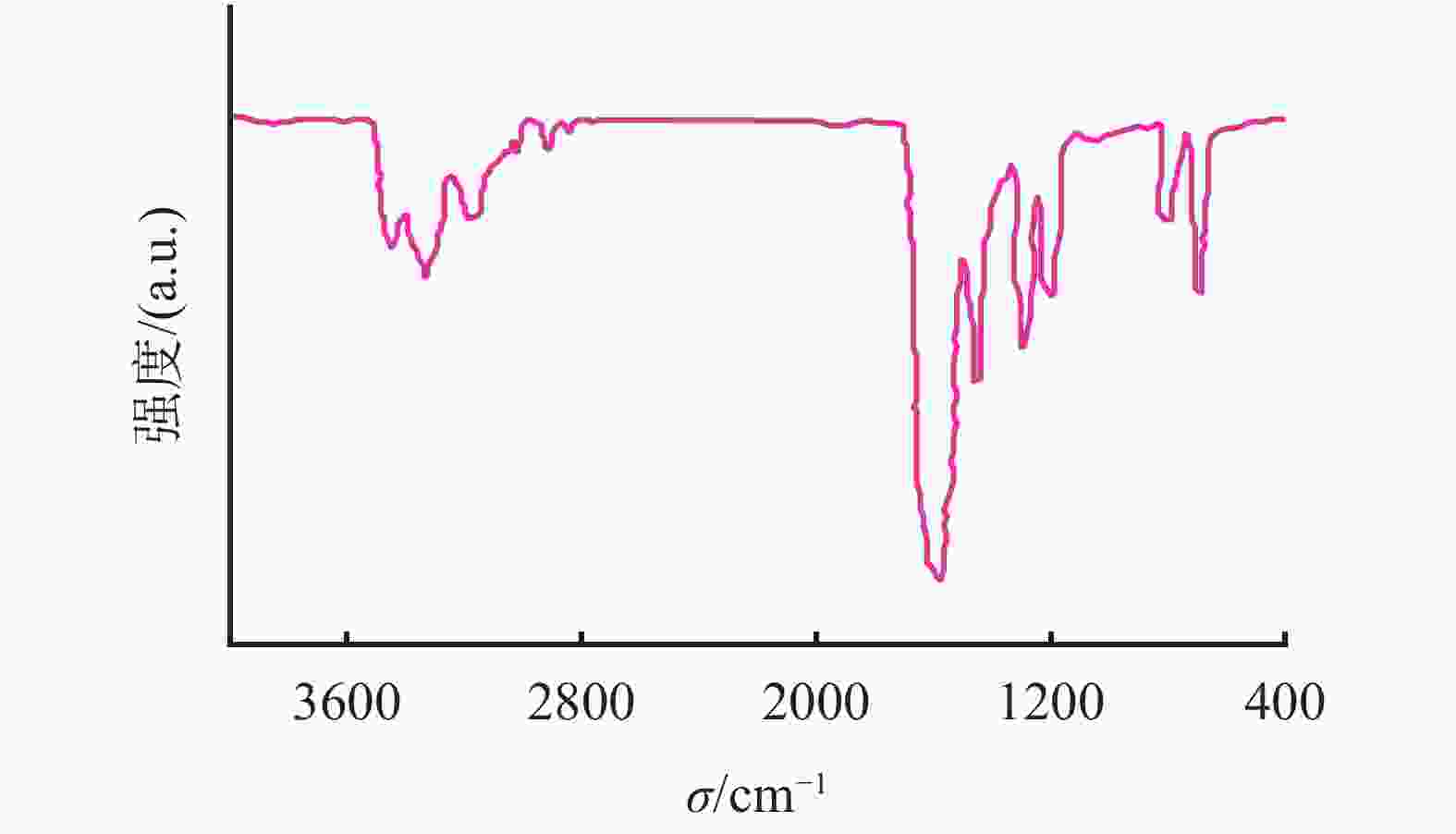
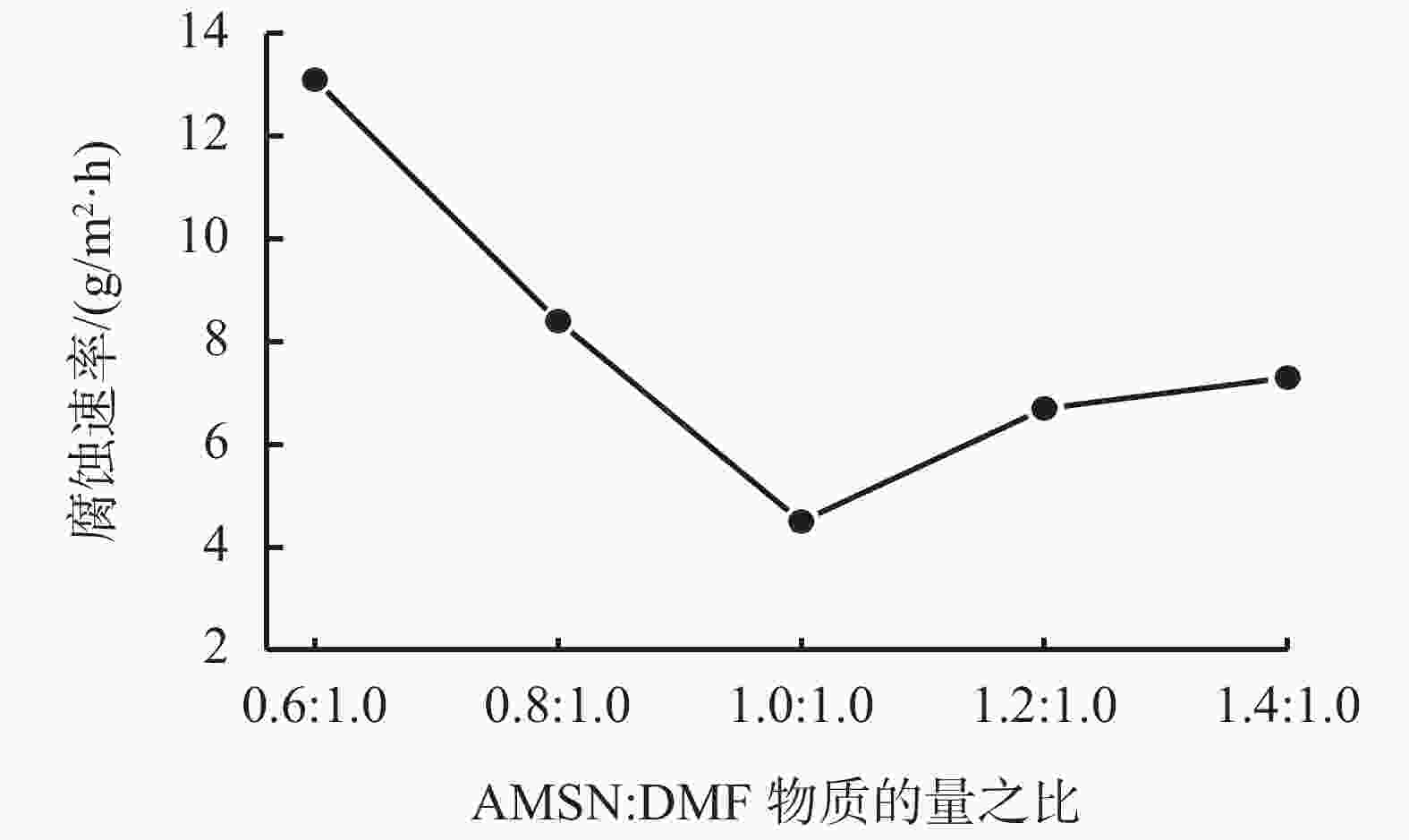
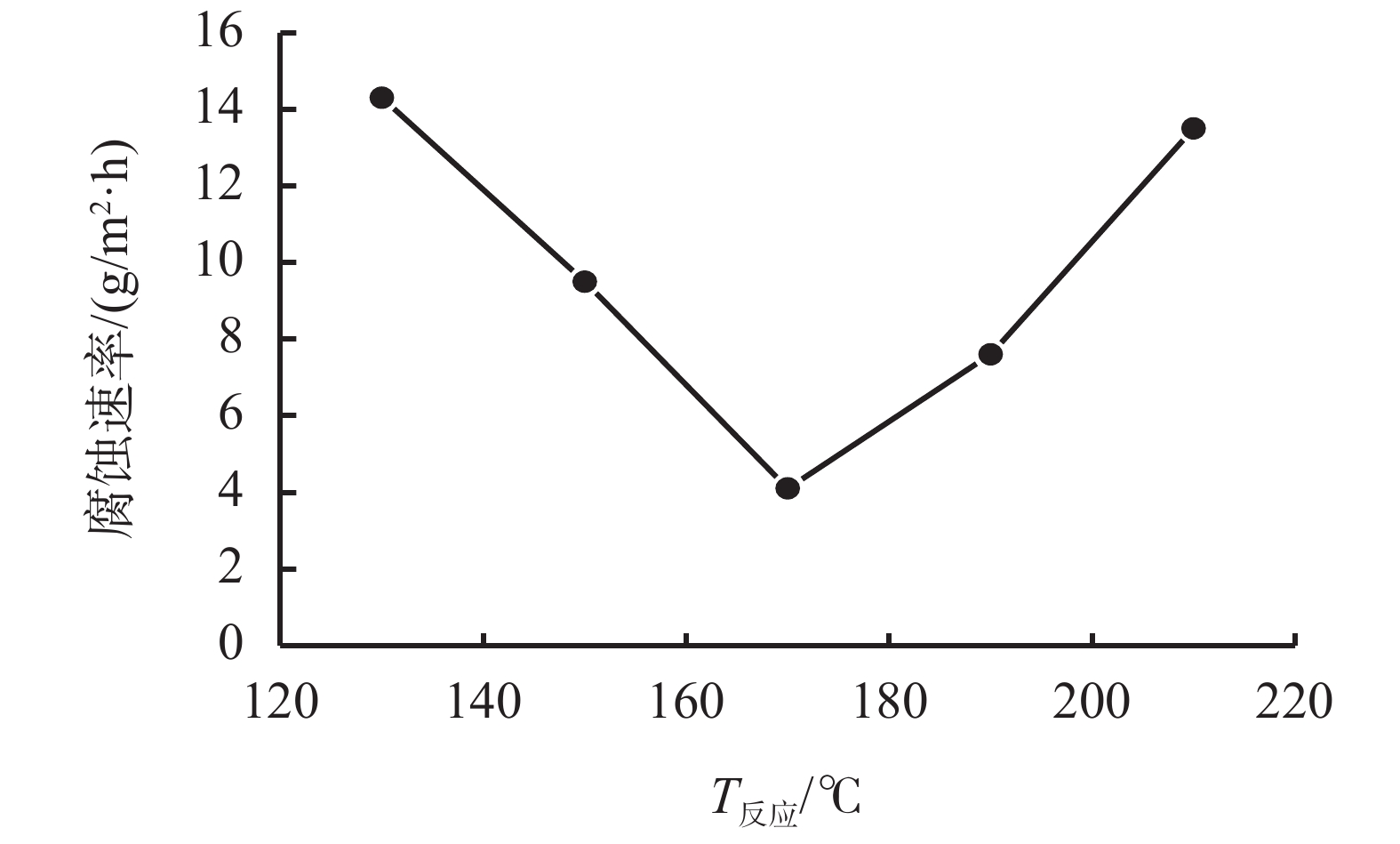
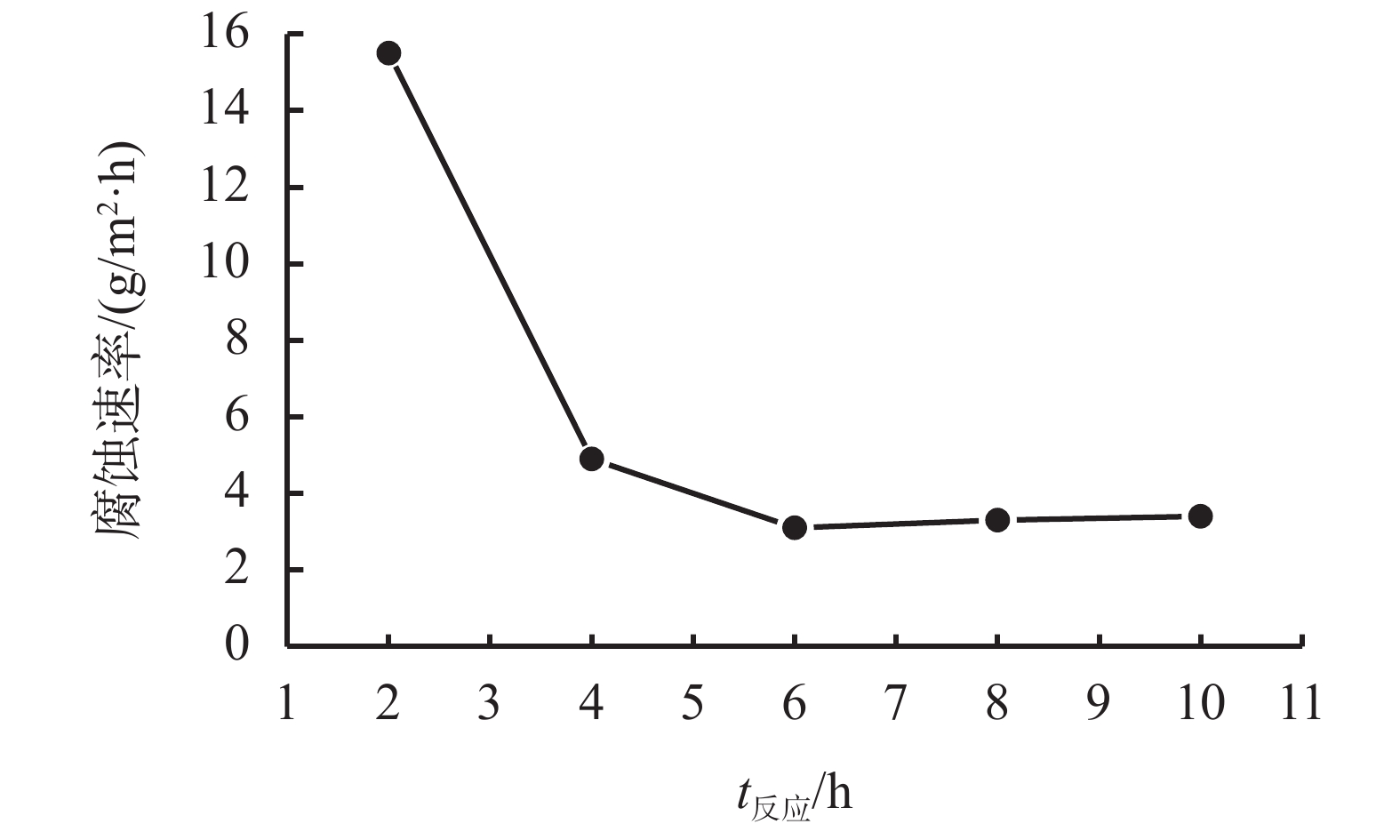
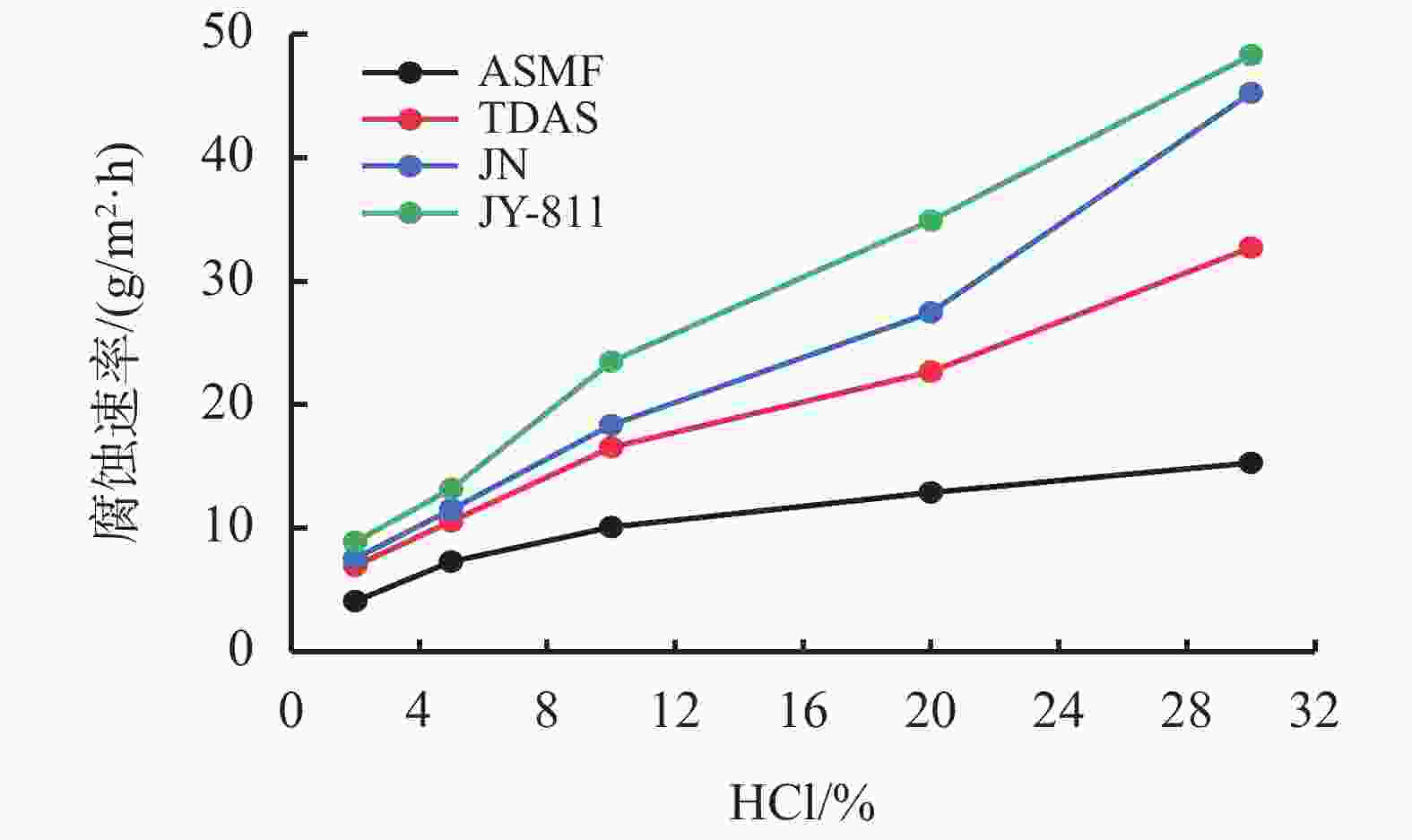
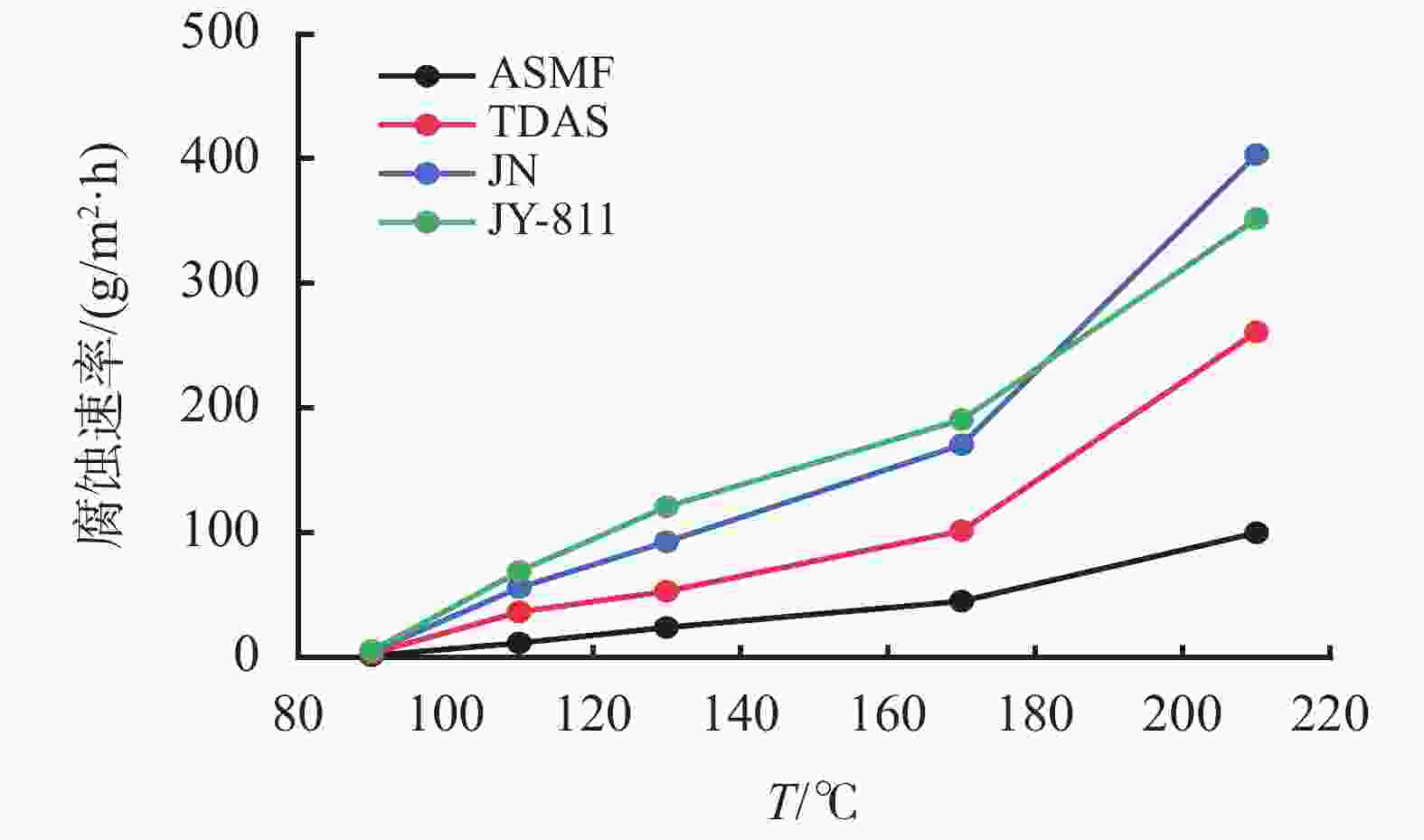
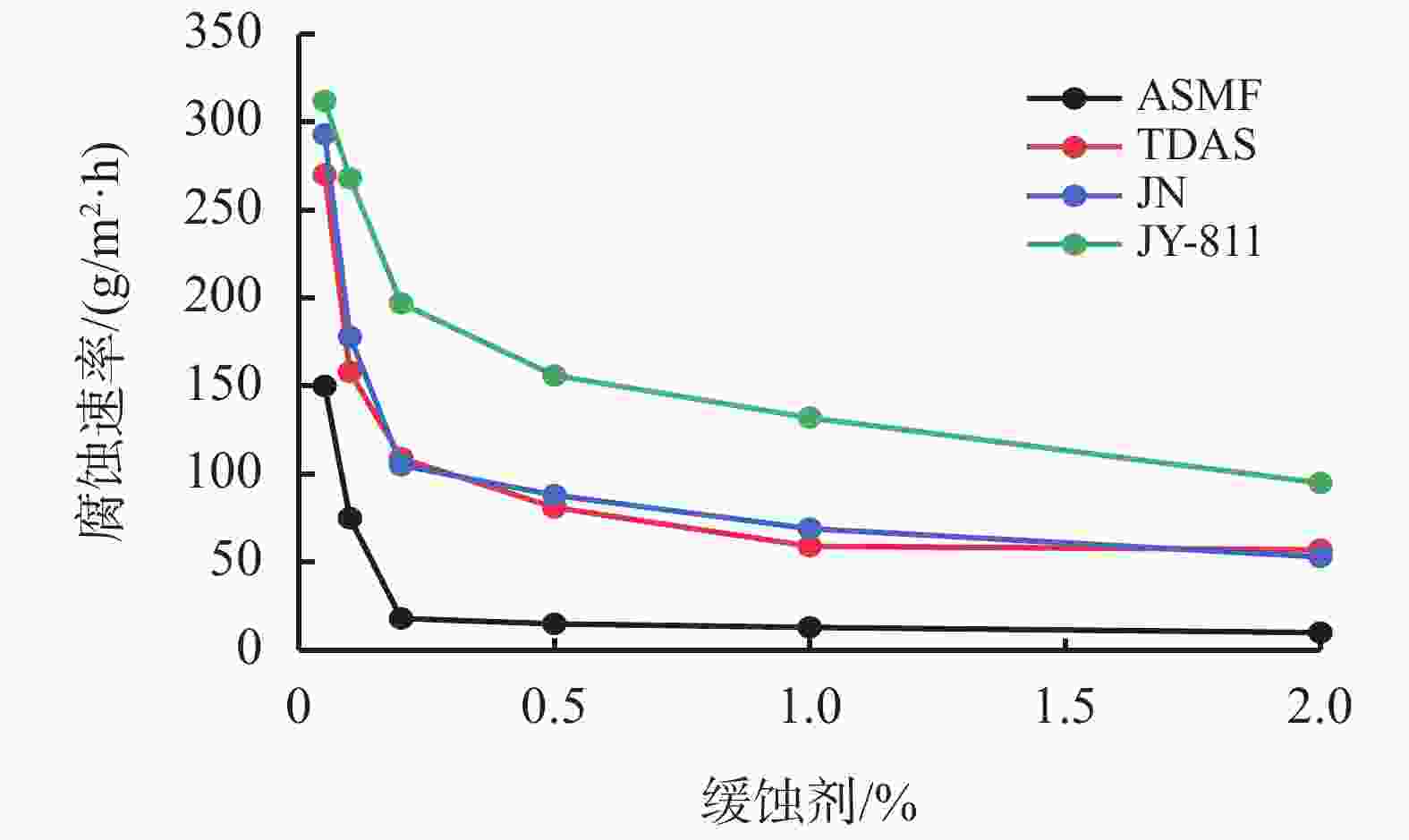
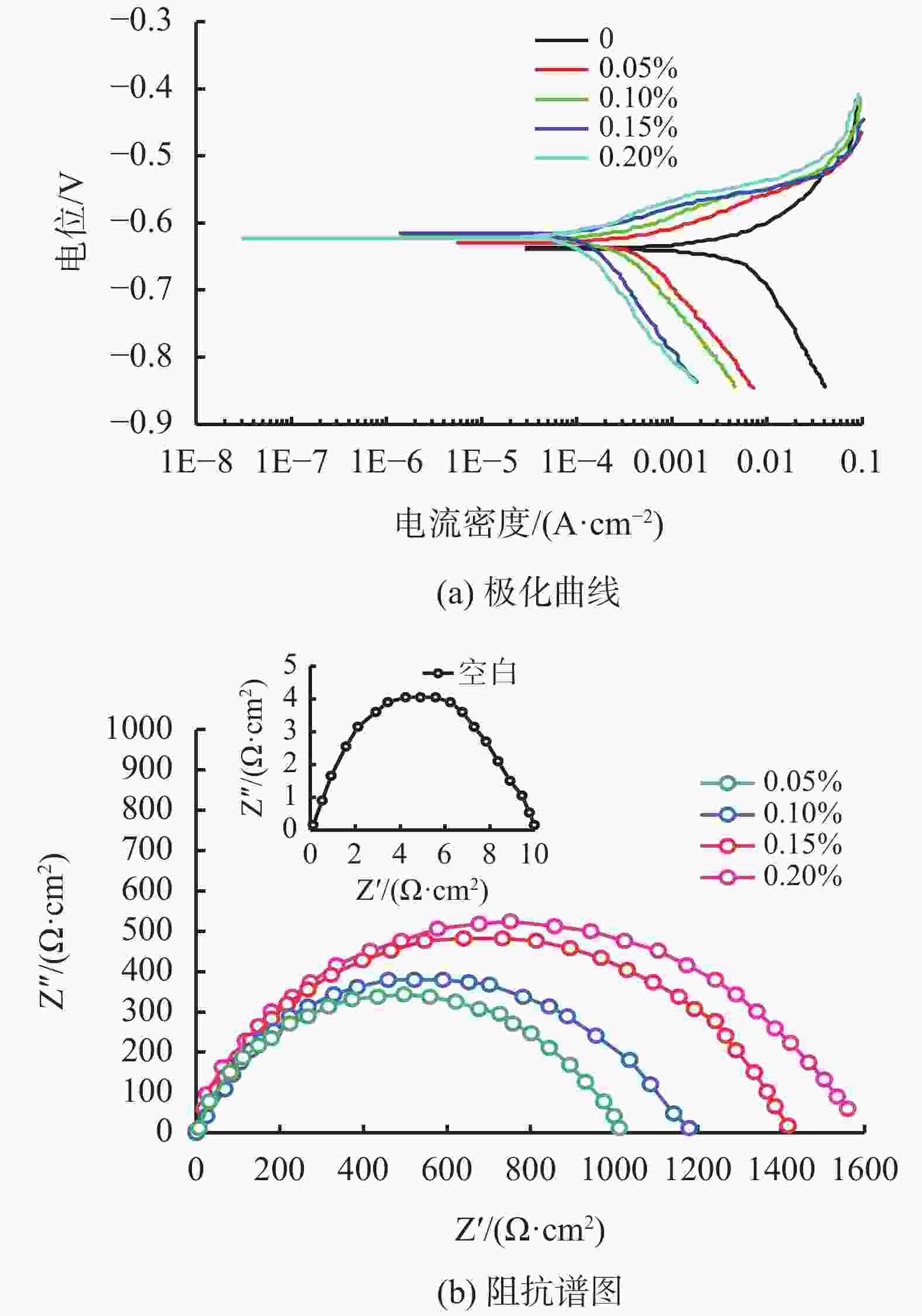
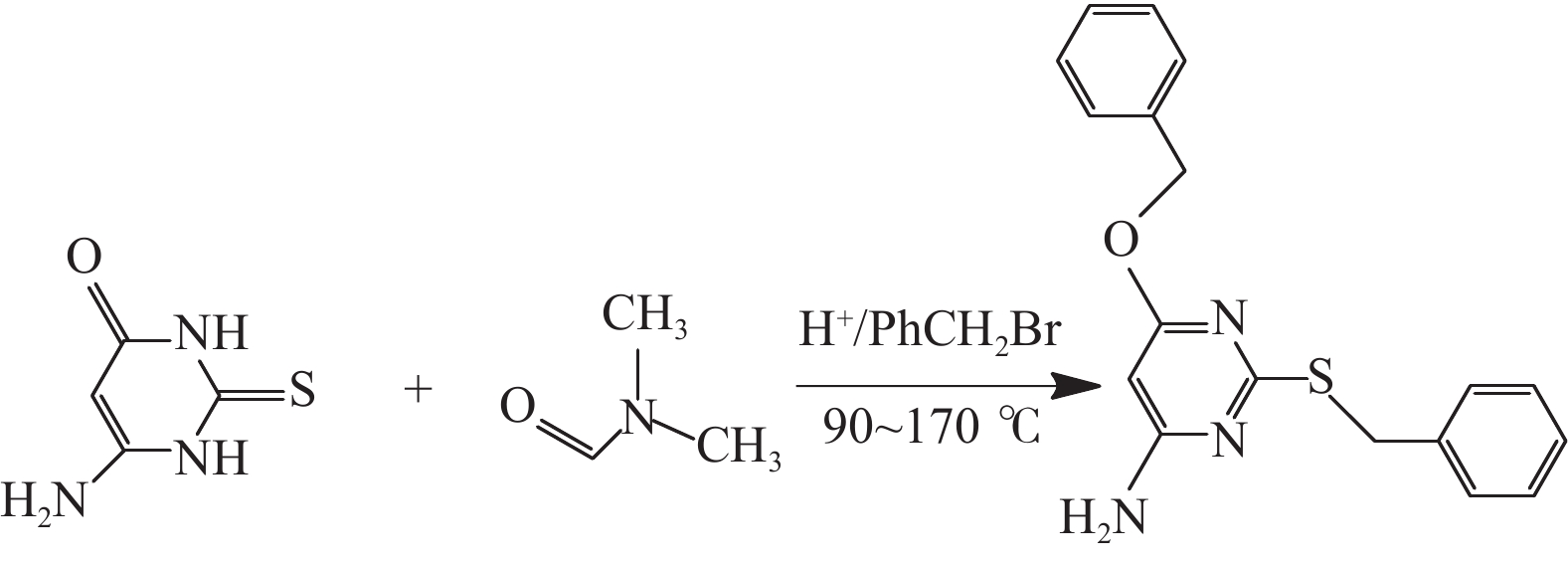
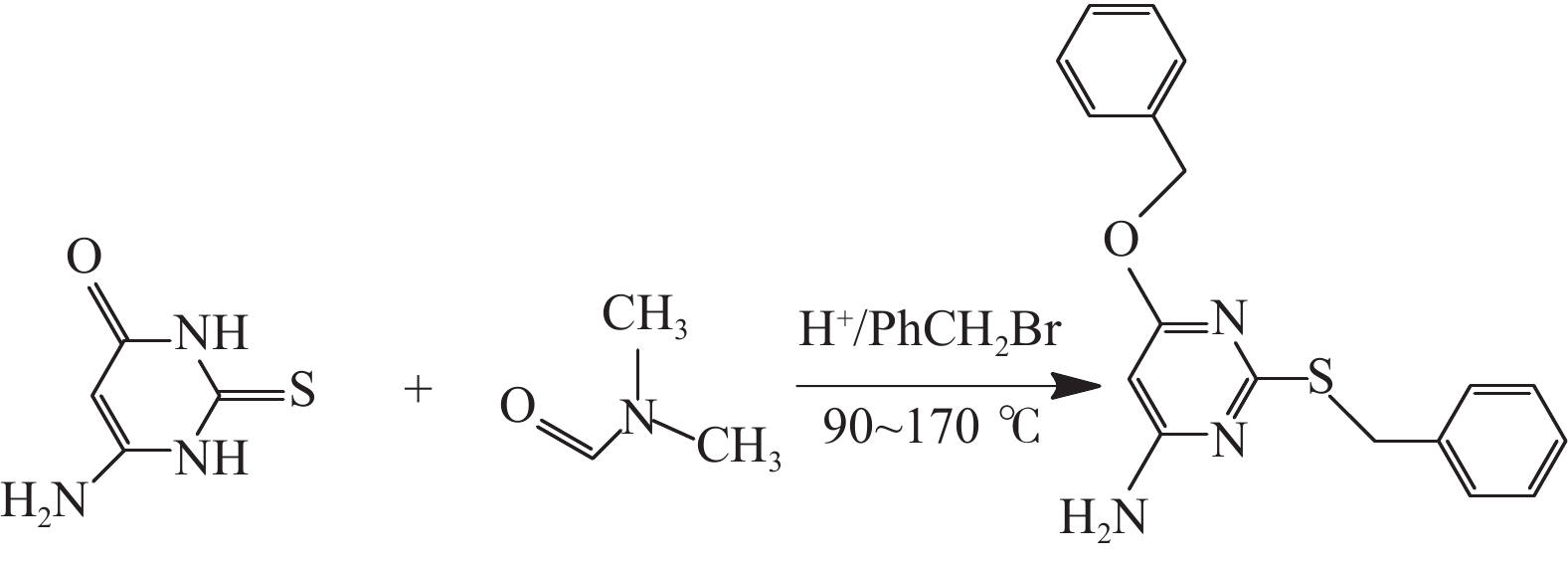
摘要: 采用绿色嘧啶生物材料4-氨基-6-羟基-2-巯基嘧啶(AMSN)和无水N,N-二甲基甲酰胺(DMF),一步合成了高效嘧啶衍生物酸化缓蚀剂ASMF,红外光谱表征及元素分析结果表明其合成纯度较高。通过研究其合成过程中影响ASMF产率的因素发现:AMSN和DMF的物质的量之比的水平宜选为1∶1,反应温度不宜高于170 ℃,反应时间至少要有4 h,ASMF可获得较高产率。通过中试生产高效嘧啶衍生物酸化缓蚀剂ASMF,并研究其缓蚀性能,与市售生物缓蚀剂相比,中试产品嘧啶衍生物酸化缓蚀剂ASMF在高浓度酸液(>20%)中仍有优秀的缓蚀性能,其耐温能达170 ℃,添加量低于0.2%时,缓蚀剂ASMF仍能表现出优异的缓蚀性能。ASMF电化学极化曲线结果表明,缓蚀剂ASMF能有效地抑制腐蚀过程中阴极和阳极过程的电极反应,是偏阴极抑制的混合型缓蚀剂;阻抗结果表明,ASMF具有良好的缓蚀性能,其在添加量为0.2%时,其缓蚀效率可达99.85%,具有高效的缓蚀效率;岩心损害评价表明,缓蚀剂ASMF的岩心伤害性能属于弱伤害,对环境友好。综上认为所研制的产品为绿色高效嘧啶衍生物酸化缓蚀剂。Abstract: Using the environmentally friendly biomaterial 4-amino-6-hydroxy-2-mercaptopyrimidine (AMSN) and the anhydrous N,N-dimethylformamide (DMF), a high performance pyrimidine derivative ASMF, which is a corrosion inhibitor for acid job, has been developed through one-step reaction. Characterization by IR spectroscopy and element analysis show that the reaction gave high purity product. Studies on the factors affecting the yield of ASMF show that the optimum molar ratio of AMSN and DMF is 1:1, the reaction temperature should not be higher than 170 °C, and the reaction time should be at least 4 h. The yield of ASMF is relatively high under these conditions. An ASMF sample produced in an experimental production showed excellent corrosion inhibitive capacity in high concentration (>20%) acid solutions, and it functioned at temperatures as high as 170 °C. ASMF shows superior corrosion inhibitive performance even at concentrations less than 0.2%. The electrochemical polarization curve of ASMF shows that ASMF can effectively inhibit the electrode reactions taken place at the cathode and anode during a corrosion process, and it is a mixed corrosion inhibitor which shows cathode inhibition property. Impedance test results show that ASMF has good corrosion inhibition efficiency, at a concentration of 0.2%, the corrosion inhibition efficiency of ASMF is 99.85%. Core damage evaluation test results show that ASMF shows only weak damage to the permeability of cores flooded with the ASMF solution, and ASMF is environmentally friendly. It can be concluded that the product developed is an environmentally friendly high performance pyrimidine derivative corrosion inhibitor for acid jobs
-
表 1 缓蚀剂ASMF的相关电化学参数拟合结果
ASMF/
%腐蚀电位/
mV腐蚀电流/
mA·cm−2ba/
mV·dec−1bc/
mV·dec−1缓蚀效率/
%空白 −765 1.3×10−5 71 −232 0.05 −717 1.8×10−7 77 −123 95.49 0.10 −695 4.3×10−8 51 −105 97.65 0.15 −691 3.7×10−8 50 −119 98.70 0.20 −689 2.1×10−8 56 −113 99.85 注:ba和bc为塔菲尔斜率。 表 2 缓蚀剂ASMF对长庆油田天然岩心的伤害评价
岩心 K0/mD Kd/mD 岩心伤害率/% C-1 0.0334 0.0283 10.78 C-2 0.0463 0.0400 9.35 Y-1 0.0375 0.0318 10.64 Y-2 0.0386 0.0330 10.09 表 3 不同缓蚀剂在长庆油田应用实验
缓蚀
剂试验
井号T井内/
℃井内酸浓度/
%油管节扣在
井下时间/d缓释效
率/%JN 1# 85~90 15.1~17.3 5 53.2 JY-811 2# 85~90 15.2~16.9 5 48.3 TDAS 3# 130~150 18.7~19.3 5 65.3 ASMF 4# 150~170 18.2~20.4 5 98.3 -
[1] 成涛,刘鹏超,欧志鹏,等. 累积工况下CO2对稠油热采水泥石的修复作用[J]. 钻井液与完井液,2021,38(5):628-633.CHENG Tao, LIU Pengchao, OU Zhipeng, et al. Remediation of set cement in heavy oil thermal production wells with CO2 under cumulative working conditions[J]. Drilling Fluid & Completion Fluid, 2021, 38(5):628-633. [2] HARUNA K, OBOT I B, ANKAH N K, et al. Gelatin: a green corrosion inhibitor for carbon steel in oil well acidizing environment[J]. Journal of Molecular Liquids, 2018, 264:515-525. doi: 10.1016/j.molliq.2018.05.058 [3] OLAJIRE A A. Corrosion inhibition of offshore oil and gas production facilities using organic compound inhibitors-A review[J]. Journal of Molecular Liquids, 2017, 248:775-808. doi: 10.1016/j.molliq.2017.10.097 [4] 果常旺,李善建,崔国涛,等. 一种酸化缓蚀剂的合成与性能评价[J]. 化工技术与开发,2022,51(09):1-5. doi: 10.3969/j.issn.1671-9905.2022.09.001GUO Changwang, LI Shanjian, CUI Guotao, et al. Synthesis and performance evaluation of an acidizing corrosion inhib-itor[J]. Technology & Development of Chemical Industry, 2022, 51(09):1-5. doi: 10.3969/j.issn.1671-9905.2022.09.001 [5] 王国瑞,刘峥,刘二喜,等. 绿色缓蚀剂研究现状与展望[J]. 腐蚀与防护,2009,30(10):732-737.WANG Guorui, LIU Zheng, LIU Erxi, et al. Research and development status of the environmental corrosion inhibitors[J]. Corrosion & Protection, 2009, 30(10):732-737. [6] 侯保山. 新型高效嘧啶类缓蚀剂的开发、缓蚀性能及理论计算[D]. 武汉: 华中科技大学, 2021.HOU Baoshan. Development, corrosion inhibition performance and theoretical calculation of novel high-efficient pyrimidine inhibitors[D]. Wuhan: Huazhong University of Science and Technology, 2021. [7] SINGH P, SINGH A, QURAISHI M A. Thiopyrimidine derivatives as new and effective corrosion inhibitors for mild steel in hydrochloric acid: Electrochemical and quantum chemical studies[J]. Journal of the Taiwan Institute of Chemical Engineers, 2016, 60:588-601. doi: 10.1016/j.jtice.2015.10.033 [8] 涂胜,汤琪,王孝华,等. 嘧啶硫酮缓蚀剂的合成及其性能评价[J]. 西南大学学报(自然科学版),2018,40(11):168-174. doi: 10.13718/j.cnki.xdzk.2018.11.022TU Sheng, TANG Qi, WANG Xiaohua, et al. Synthesis of pyrimidin thione and evaluation of its corrosion inhibition[J]. Journal of Southwest University (Natural Science Edition) , 2018, 40(11):168-174. doi: 10.13718/j.cnki.xdzk.2018.11.022 [9] 范雨. 一种硝酸缓蚀剂合成及性能评价[D]. 西南石油大学, 2014.FAN Yu. Synthesis and performance evaluation of a nitric acid corrosion inhibitor[D]. Chengdu: Southwest Petroleum University, 2014. [10] REZNIK V S, AKAMSIN V D, KHODYREV Y P, et al. Mercaptopyrimidines as inhibitors of carbon dioxide corrosion of iron[J]. Corrosion science, 2008, 50(2):392-403. doi: 10.1016/j.corsci.2007.06.021 [11] 张兴德,原励,王川,等. 一种耐200 ℃ 高温缓蚀剂[J]. 钻井液与完井液,2020,37(5):664-669.ZHANG Xingde, YUAN Li, WANG Chuan, et al. A high temperature corrosion inhibitor for use at 200 ℃[J]. Drilling Fluid & Completion Fluid, 2020, 37(5):664-669. [12] OU H H, TRAN Q T P, LIN P H. A synergistic effect between gluconate and molybdate on corrosion inhibition of recirculating cooling water systems[J]. Corrosion Science, 2018, 133:231-239. doi: 10.1016/j.corsci.2018.01.014 [13] HE X, MAO J, MA Q, et al. Corrosion inhibition of perimidine derivatives for mild steel in acidic media: electrochemical and computational studies[J]. Journal of Molecular Liquids, 2018, 269:260-268. doi: 10.1016/j.molliq.2018.08.021 [14] EL FAYDY M, TOUIR R, TOUHAMI M E, et al. Corrosion inhibition performance of newly synthesized 5-alkoxymethyl-8-hydroxyquinoline derivatives for carbon steel in 1 M HCl solution: experimental, DFT and Monte Carlo simulation studies[J]. Physical Chemistry Chemical Physics, 2018, 20(30):20167-20187. doi: 10.1039/C8CP03226B [15] PAREEK S, JAIN D, HUSSAIN S, et al. A new insight into corrosion inhibition mechanism of copper in aerated 3.5 wt. % NaCl solution by eco-friendly Imidazopyrimidine Dye: experimental and theoretical approach[J]. Chemical Engineering Journal, 2019, 358:725-742. doi: 10.1016/j.cej.2018.08.079 [16] EDWARDS H G M, JOHNSON A F, LAWSON E E. Structural determination of substituted mercaptothiadiazoles using FT-Raman and FT-IR spectroscopy[J]. Journal of molecular structure, 1995, 351(94):51-63. [17] 李向红. 嘧啶衍生物对冷轧钢在 HCI 和 H2SO4 介质中的缓蚀性能及机理研究[D]. 云南大学, 2015.LI Xianghong. Inhibition effect and mechanism of pyrimidine derivatives on the corrosion of cold rolled steel in HCl and H2SO4 solutions[D]. Kunming: Yunnan University, 2015. [18] 薛丹,张硕硕,朱明明,等. 一种新型高温酸化缓蚀剂的制备及性能[J]. 腐蚀与防护,2022,43(3):36-41. doi: 10.11973/fsyfh-202203006XUE Dan, ZHANG Shuoshuo, ZHU Mingming, et al. Preparation and properties of a new high-temperature acidified corrosion inhibitor[J]. Corrosion & Protection, 2022, 43(3):36-41. doi: 10.11973/fsyfh-202203006 [19] 仝方超,李善建,董晓军,等. 一种油田酸化用醛酮胺缓蚀剂的合成及缓蚀机理研究[J]. 西安石油大学学报(自然科学版),2015,30(4):87-90.TONG Fangchao, LI Shanjian, DONG Xiaojun, et al. Synthesis and Corrosion Inhibition Mechanism of an Aldehyde-Ketamine Corrosion Inhibitor for Oilfield Acidification[J]. Journal of Xi'an Shiyou University (Natural Science Edition) , 2015, 30(4):87-90. [20] GUO L, ZHU S, ZHANG S. Experimental and theoretical studies of benzalkonium chloride as an inhibitor for carbon steel corrosion in sulfuric acid[J]. Journal of Industrial and Engineering Chemistry, 2015, 24:174-180. doi: 10.1016/j.jiec.2014.09.026 [21] GITE V V, TATIYA P D, MARATHE R J, et al. Microencapsulation of quinoline as a corrosion inhibitor in polyurea microcapsules for application in anticorrosive PU coatings[J]. Progress in Organic Coatings, 2015, 83:11-18. doi: 10.1016/j.porgcoat.2015.01.021 [22] SOLMAZ R, KARDAŞ G, ÇULHA M, et al. Investigation of adsorption and inhibitive effect of 2-mercaptothiazoline on corrosion of mild steel in hydrochloric acid media[J]. Electrochimica Acta, 2008, 53(20):5941-5952. doi: 10.1016/j.electacta.2008.03.055 [23] 曹楚南. 腐蚀电化学原理[M]. (第三版). 北京: 化学工业出版社, 2008: 165-165.CAO Chunan. Principles of electrochemistry of corrosion[M]. Beijing: Chemical Industry Press, 2008: 165-165. -




 下载:
下载: