Study on Solubilization Behavior of Molecular Simulation Cosolvent in Siloxane SC-CO2 Fracturing Fluid
-
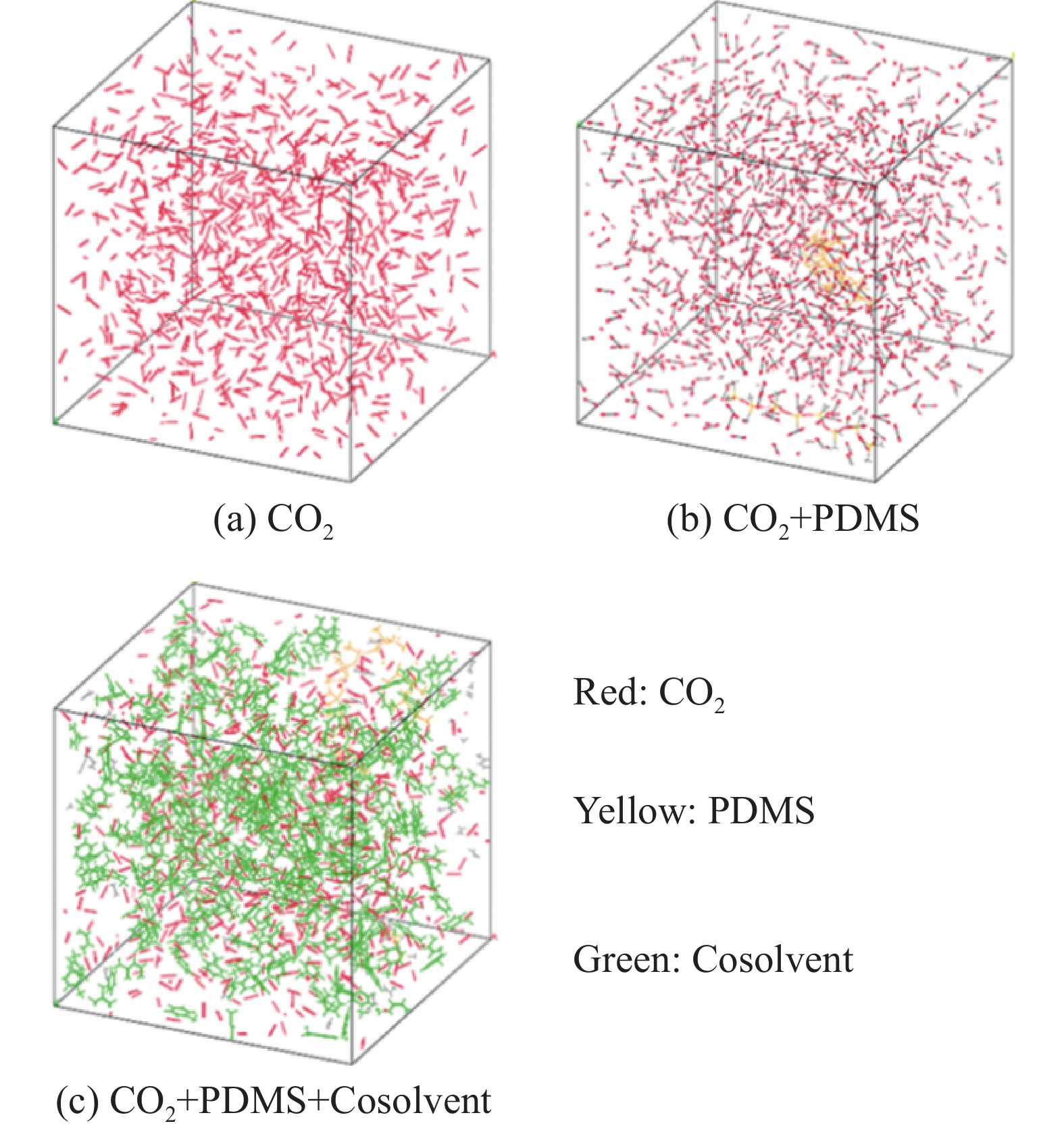
摘要: 超临界二氧化碳压裂液体系由于黏度低,一般选用加入增稠剂的方法来克服携砂效率低的难题。硅氧烷类增稠剂具有低内聚能和良好的增黏性被广泛地选用,但是使用时需要添加助溶剂提高溶解效果。因此,选用被广泛使用的聚二甲基硅氧烷(PDMS)作为研究对象,利用分子模拟研究了甲醇、甲苯和环己烷等助溶剂的加入对聚二甲基硅氧烷在SC-CO2体系中溶解行为的影响。基于溶剂-溶剂和溶剂-溶质的结合能、径向分布函数和内聚能密度等参数,对比分析了极性助溶剂和非极性助溶剂对聚二甲基硅氧烷在超临界二氧化碳压裂液体系中的助溶效果。分子模拟结果表明,在相同助溶剂含量下,甲醇与溶剂体系溶解度参数差值小于0.5,助溶效果优于甲苯和环己烷。结论分析认为,使用助溶剂提高PDMS在SC-CO2中溶解度的实质是CO2与PDMS聚合物分子间作用力、CO2与助溶剂分子间作用力以及PDMS聚合物与助溶剂分子间作用力的平衡。因此,当硅氧烷类增稠剂本身为非极性材料时,推荐采用甲苯作为助溶剂。若硅氧烷类材料具有一定弱极性时,采用甲醇最为适合。Abstract: Due to the low viscosity of supercritical carbon dioxide fracturing fluid system, the method of adding tackifier is generally used to overcome the problem of low sand carrying efficiency. Siloxane tackifiers are widely used due to their low cohesive energy and good tackifying effect, but it is necessary to add cosolvent to improve the dissolution effect. Therefore, in this paper, the widely used polydimethylsiloxane was selected as the research object, and the effect of the addition of cosolvents such as methanol, toluene and cyclohexane on the dissolution behavior of polydimethylsiloxane in SC-CO2 system was studied by molecular simulation. Based on the binding energy, cohesive energy density and radial distribution function of solvent-solvent and solvent-solute, the solubilization effect of polar cosolvent and non-polar cosolvent on polydimethylsiloxane in supercritical carbon dioxide fracturing fluid system was compared and analyzed. The molecular simulation results show that the solubility parameter difference between methanol and solvent system is less than 0.5 at the same cosolvent content, and the solubilization effect is better than that of toluene and cyclohexane. The conclusion is that the essence of using cosolvent to improve the solubility of PDMS in SC-CO2 is the balance of the intermolecular force between CO2 and PDMS polymer, the intermolecular force between CO2 and cosolvent, and the intermolecular force between PDMS polymer and cosolvent. Therefore, when the siloxane tackifier itself is a non-polar material, toluene is recommended as a cosolvent. When the siloxane material has a certain weak polarity, methanol is the most suitable.
-
Key words:
- SC-CO2 fracturing fluid /
- PDMS /
- Molecular simulation /
- Cosolvent /
- Viscosities
-
表 1 分子动力学计算系统的构成
体系 组成 PDMS
分子链数助溶剂
分子数CO2
分子数1 CO2 0 0 1000 2 甲苯 0 100 0 3 甲醇 0 100 0 4 环己烷 0 100 0 5 CO2/甲苯 0 100 1000 6 CO2/甲醇 0 100 1000 7 CO2/环己烷 0 100 1000 8 PDMS 2 0 0 9 CO2/PDMS 2 0 1000 10 CO2/甲苯/PDMS 2 100 1000 11 CO2/甲醇/PDMS 2 100 1000 12 CO2/环己烷/PDMS 2 100 1000 表 2 318 K下超临界CO2溶解度参数的力场验证
P/MPa P实验值/ ((MPa)1/2) P模拟值/((MPa)1/2) 误差/% 10 7.7 8.736 13.45 14 12.6 12.256 2.73 20 14.3 13.682 4.32 25 15.0 14.416 3.89 30 15.6 15.207 2.52 40 16.4 15.653 4.55 表 3 CO2与PDMS的内聚能密度与溶解度参数
体系组成 Evan/(J/m3) Eelect/(J/m3) Eother/(J/m3) CED/(J/m3) δ/(J/m3)1/2 Δδ/(J/m3)1/2 CO2 1.88×108 1.75×107 3.66×106 3.66×108 19.13 0 PDMS 2.34×106 6.08×103 3.52×104 2.39×106 1.545 17.59 CO2+PDMS 2.09×108 1.76×108 4.42×106 3.89×108 19.73 0.60 表 4 CO2与不同助溶剂的结合能
体系组成 Eall/
kJ/molEcos/
kJ/molECO2/
kJ/molEinter/
kJ/molCO2/甲苯 −911.49 738.75 −490.36 −1159.88 CO2/甲醇 −884.74 654.40 −909.02 −1139.36 CO2/环己烷 −1845.68 −510.13 −502.59 −2858.40 表 5 CO2与不同助溶剂的内聚能密度和溶解度参数
体系组成 Evan/(J/m3) Eelect/(J/m3) Eother/(J/m3) CED/(J/m3) δ/(J/m3)1/2 Δδ/(J/m3)1/2 CO2 1.88×108 1.75×107 3.66×106 3.66×108 19.13 0 甲苯 1.43×107 9.06×105 3.25×105 1.56×107 3.94 15.19 甲醇 0.33×108 2.99×108 4.22×104 3.32×107 5.76 13.37 环己烷 1.21×107 1.35×104 2.53×105 1.24×107 3.52 15.61 CO2+甲苯 2.04×108 1.08×108 4.15×106 3.16×108 17.77 1.36 CO2+甲醇 1.63×108 1.66×108 3.41×106 3.33×108 18.24 0.89 CO2+环己烷 1.73×108 8.25×107 3.53×106 2.59×108 16.09 3.04 表 6 SC-CO2、PDMS与不同助溶剂混溶体系的结合能
体系组成 Eall/kJ/mol ECO2/kJ/mol EPDMS/kJ/mol Ecos/kJ/mol Einter/kJ/mol CO2+PDMS −4613.80 −1412.85 −3011.84 0 −188.12 CO2+PDMS+甲苯 −3937.61 −317.64 −2973.87 682.27 −1328.37 CO2+PDMS+甲醇 −3995.30 −769.23 −2984.61 584.92 −826.38 CO2+PDMS +环己烷 −4854.87 −416.01 −2956.41 −521.16 −964.29 表 7 SC-CO2、PDMS与不同助溶剂混溶体系的结合能密度与溶解参数
体系组成 Evan/(J/m3) Eelect/(J/m3) Eother/(J/m3) CED/(J/m3) δ/(J/m3)1/2 Δδ/(J/m3)1/2 CO2+PDMS 2.09×108 1.76×107 4.42×106 3.89×108 19.73 0 CO2+PDMS+甲苯 2.02×108 1.09×108 4.32×106 3.14×108 17.73 2.00 CO2+PDMS+甲醇 1.83×108 1.83×108 3.99×106 3.70×108 19.25 0.48 CO2+PDMS+环己烷 1.79×108 8.50×107 3.79×106 2.68×108 16.37 3.36 -
[1] 王海柱,李根生,贺振国,等. 超临界CO2岩石致裂机制分析[J]. 岩土力学,2018,39(10):3589-3596.WANG Haizhu, LI Gensheng, HE Zhenguo, et al. Analysis of mechanisms of supercritical CO2 fracturing[J]. Rock and Soil Mechanics, 2018, 39(10):3589-3596. [2] 赵金洲,任岚,蒋廷学,等. 中国页岩气压裂十年: 回顾与展望[J]. 天然气工业,2021,41(8):121-142.ZHAO Jinzhou, REN Lan, JIANG Tingxue, et al. Ten years of gas shale fracturing in China: review and prospect[J]. Natural Gas Industry, 2021, 41(8):121-142. [3] 郑永,王海柱,李根生,等. 超临界CO2压裂迂曲裂缝内支撑剂运移特征[J]. 天然气工业,2022,42(3):71-80.ZHENG Yong, WANG Haizhu, LI Gensheng, et al. Proppant transport characteristics in tortuous fractures induced by supercritical CO2 fracturing[J]. Natural Gas Industry, 2022, 42(3):71-80. [4] HELLER J P, DANDGE D K, CARD R J, et al. Direct thickeners for mobility control of CO2 floods[J]. Society of Petroleum Engineers Journal, 1985, 25(5):679-686. doi: 10.2118/11789-PA [5] KILIC S, MICHALIK S, WANG Y, et al. Phase behavior of oxygen-containing polymers in CO2[J]. Macromolecules, 2007, 40(4):1332-1341. doi: 10.1021/ma061422h [6] XU J H, WLASCHIN A, ENICK R M. Thickening carbon dioxide with the fluoroacrylate-styrene copolymer[J]. SPE Journal, 2003, 8(2):85-91. doi: 10.2118/84949-PA [7] DU M Y, SUN X, DAI C L, et al. Laboratory experiment on a toluene-polydimethyl silicone thickened supercritical carbon dioxide fracturing fluid[J]. Journal of Petroleum Science and Engineering, 2018, 166:369-374. doi: 10.1016/j.petrol.2018.03.039 [8] ALZOBAIDI S, LEE J, JIRIES S, et al. Carbon dioxide-in-oil emulsions stabilized with silicone-alkyl surfactants for waterless hydraulic fracturing[J]. Journal of Colloid and Interface Science, 2018, 526:253-267. doi: 10.1016/j.jcis.2018.04.056 [9] LIU B, WANG Y L, LIANG L. Preparation and performance of supercritical carbon dioxide thickener[J]. Polymers, 2020, 13(1):78. doi: 10.3390/polym13010078 [10] 沈子齐,王彦玲,贾文峰,等. 新型低渗储层CO2增稠剂的静态悬砂性能及悬砂机理分析[J]. 应用化工,2022,51(1):68-72.SHEN Ziqi, WANG Yanling, JIA Wenfeng, et al. Static suspended sand performance and mechanism analysis of new CO2 thickener for low permeability reservoir[J]. Applied Chemical Industry, 2022, 51(1):68-72. [11] 赵明伟,李阳,高明伟,等. 超临界二氧化碳溶解与增稠性能的综合性实验设计[J]. 实验技术与管理,2021,38(1):78-81. doi: 10.16791/j.cnki.sjg.2021.01.017ZHAO Mingwei, LI Yang, GAO Mingwei, et al. Comprehensive experimental design of solubility and thickening properties of supercritical carbon dioxide[J]. Experimental Technology and Management, 2021, 38(1):78-81. doi: 10.16791/j.cnki.sjg.2021.01.017 [12] ZHANG J, XIAO B, ZHANG G X, et al. A New thickener for CO2 anhydrous fracturing fluid[J]. MATEC Web of Conferences, 2015, 31: 01002. [13] SANG Q, ZHAO X Y, ABDELFATAH E, et al. Dispersibility of poly (vinyl acetate) modified silica nanoparticles in carbon dioxide with several cosolvents[J]. Langmuir, 2021, 37(2):655-665. doi: 10.1021/acs.langmuir.0c02522 [14] ZHAO M W, LI Y, GAO M W, et al. Formulation and performance evaluation of polymer-thickened supercritical CO2 fracturing fluid[J]. Journal of Petroleum Science and Engineering, 2021, 201:108474. doi: 10.1016/j.petrol.2021.108474 [15] LI B F, LIU K, ZHU J, et al. Cosolvent Effect on the Solubility of Ammonium Benzoate in Supercritical Carbon Dioxide[J]. Journal of Chemical & Engineering Data, 2022, 67(3):689-694. [16] HU D D, SUN S J, YUAN P Q, et al. Evaluation of CO2-philicity of poly (vinyl acetate) and poly (vinyl acetate-alt-maleate) copolymers through molecular modeling and dissolution behavior measurement[J]. The Journal of Physical Chemistry B, 2015, 119(7):3194-3204. doi: 10.1021/jp5130052 [17] GONG H J, ZHANG H, XU L, et al. Effects of cosolvent on dissolution behaviors of PVAc in supercriticalCO2: A molecular dynamics study[J]. Chemical Engineering Science, 2019, 206:22-30. doi: 10.1016/j.ces.2019.05.023 [18] 陈睿,范宏. 超支化含苯基聚硅氧烷亲CO2特性及分子模拟[J]. 精细化工,2020,37(6):1282-1288.CHEN Rui, FAN Hong. CO2-philic properties of hyperbranched phenyl-containing polysiloxanes with molecular simulation[J]. Fine Chemicals, 2020, 37(6):1282-1288. [19] XUE P, SHI J, CAO X L, et al. Molecular dynamics simulation of thickening mechanism of supercritical CO2 thickener[J]. Chemical Physics Letters, 2018, 706:658-664. doi: 10.1016/j.cplett.2018.07.006 [20] O'BRIEN M J, PERRY R J, DOHERTY M D, et al. Anthraquinone siloxanes as thickening agents for supercritical CO2[J]. Energy & Fuels, 2016, 30(7):5990-5998. [21] LEE J J, CUMMINGS S D, BECKMAN E J, et al. The solubility of low molecular weight Poly (Dimethyl siloxane) in dense CO2 and its use as a CO2-philic segment[J]. The Journal of Supercritical Fluids, 2017, 119:17-25. doi: 10.1016/j.supflu.2016.08.003 [22] LUO Hui, WANG Rui, FAN Weiyu, et al. Calculation of solubility parameters of supercritical CO2 by molecular dynamics simulation[J]. Acta Petrolei Sinica (Petroleum Processing Section) , 2015, 31(1):78-83. [23] LIU B, WANG Y L, LIANG L, et al. Achieving solubility alteration with functionalized polydimethylsiloxane for improving the viscosity of supercritical CO2 fracturing fluids[J]. RSC Advances, 2021, 11(28):17197-17205. doi: 10.1039/D1RA02069B [24] HU D D, SUN S J, YUAN P Q, et al. Exploration of CO2-philicity of poly (vinyl acetate-co-alkyl vinyl ether) through molecular modeling and dissolution behavior measurement[J]. The Journal of Physical Chemistry B, 2015, 119(38):12490-12501. doi: 10.1021/acs.jpcb.5b08393 [25] KONG W X, LV B H, JING G H, et al. How to enhance the regenerability of biphasic absorbents for CO2 capture: An efficient strategy by organic alcohols activator[J]. Chemical Engineering Journal, 2022, 429:132264. doi: 10.1016/j.cej.2021.132264 [26] ZHANG L, LIU C, LI Q B. Molecular simulations of competitive adsorption behavior between CH4-C2H6 in K-illite clay at supercritical conditions[J]. Fuel, 2020, 260:116358. doi: 10.1016/j.fuel.2019.116358 [27] WU H, ZHANG X F, XU D, et al. Enhancing the interfacial stability and solvent-resistant property of PDMS/PES composite membrane by introducing a bifunctional aminosilane[J]. Journal of Membrane Science, 2009, 337(1–2):61-69. doi: 10.1016/j.memsci.2009.03.043 [28] TUBMAN N M, LIBERATORE E, PIERLEONI C, et al. Molecular-atomic transition along the deuterium Hugoniot curve with coupled electron-ion Monte Carlo simulations[J]. Physical Review Letters, 2015, 115(4):045301. doi: 10.1103/PhysRevLett.115.045301 [29] CHANG K S, CHUNG Y C, YANG T H, et al. Free volume and alcohol transport properties of PDMS membranes: Insights of nano-structure and interfacial affinity from molecular modeling[J]. Journal of Membrane Science, 2012, 417/418:119-130. [30] HOOVER W G. Canonical dynamics: equilibrium phase-space distributions[J]. Physical Review A, 1985, 31(3): 1695-1697. [31] BERENDSEN H J C, POSTMA J P M, VAN GUNSTEREN W F, et al. Molecular dynamics with coupling to an external bath[J]. The Journal of Chemical Physics, 1984, 81(8): 3684-3690. [32] KARASAWA N, GODDARD W A III. Acceleration of convergence for lattice sums[J]. The Journal of Physical Chemistry, 93(21), 7320-7327. [33] OUGIYANAGI J, MEGURO Y, YOSHIDA Z, et al. Solvent effect on distribution ratio of Pd (II) in supercritical carbon dioxide extraction and solvent extraction using 2-methyl-8-quinolinol[J]. Talanta, 2003, 59(6):1189-1198. doi: 10.1016/S0039-9140(03)00031-6 [34] ALLEN M P, TILDESLEY D J. Computer simulation of liquids[M]. 2nd ed. Oxford: Oxford University Press, 2017. [35] BARA J E, CARLISLE T K, GABRIEL C J, et al. Guide to CO2 separations in imidazolium-based room-temperature ionic liquids[J]. Industrial & Engineering Chemistry Research, 2009, 48(6):2739-2751. [36] WILLIAMS L L, RUBIN J B, EDWARDS H W. Calculation of hansen solubility parameter values for a range of pressure and temperature conditions, including the supercritical fluid region[J]. I ndustrial & Engineering Chemistry Research, 2004, 43(16):4967-4972. -





 下载:
下载: