Study and Evaluation of Highly Permeable Retarded Acids
-
摘要: 在碳酸盐岩储层的酸化压裂改造过程中,酸液质量的优劣直接影响着最终的改造效果。目前,常用的酸液体系存在渗透性差、腐蚀高、缓速效果不佳等问题。通过室内实验,研选出了适宜的复合有机缓速酸主体酸液以及酸用降阻剂、缓蚀剂和铁离子稳定剂等助剂,开发出了一套适合碳酸盐岩储层的新型强渗透缓速酸液体系。实验室综合评价结果表明,该强渗透缓速酸液体系具有良好的缓速性能、较低的腐蚀速率以及更优的渗透性能。此项研究对碳酸盐岩油藏储量的充分动用及高效开发具有重要的理论指导意义和实际应用价值。Abstract: In acid fracturing carbonate reservoirs, the quality of the acid directly affects the quality of the stimulation. Problems with the acids presently used in fracturing operation include low permeability, high corrosion rate and poor retarding performance. To solve these problems, an acid used as the main component of a compound organic retarded acids, a drag reducer for acidizing operation, a corrosion inhibitor and an iron ion stabilizer were selected to formulate a new high permeability retarded acid suitable for use in acidizing carbonate reservoirs. Laboratory experimental results showed that this new retarded acid has good retarding performance, low corrosion rate and better permeating ability. This study is of theoretical guiding significance and practical application value for fully and efficiently developing carbonate reservoirs.
-
Key words:
- Carbonate reservoir /
- High permeability acid /
- Organic retarded acid /
- Acid-rock reaction
-
表 1 90 ℃下不同酸液的缓速能力
酸液配比 反应5 min
消耗质量/g缓速
率/%36 h溶蚀
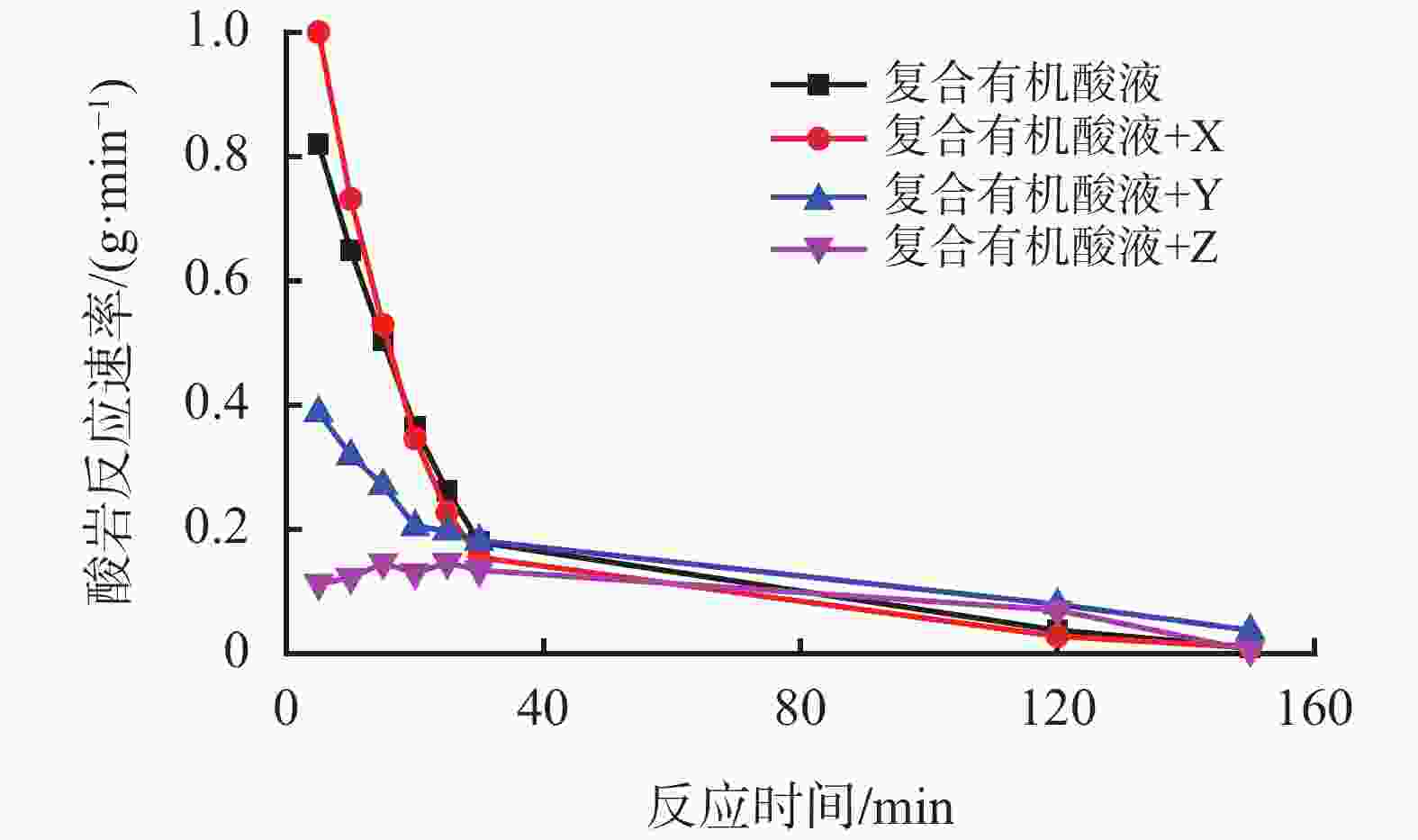
质量/g15.0 % HCl 27.63 38.24 草酸+10.0% HCl 3.84 86.10 18.45 乳酸+10.0% HCl 3.56 87.12 19.29 有机酸A +10.0 % HCl 3.07 88.89 23.61 有机酸B +10.0% HCl 2.43 91.21 24.74 复合有机酸液 1.29 95.33 28.45 注:复合有机酸液:有机酸A+有机酸B+10.0%HCl 表 2 加入不同缓速剂的酸液体系溶蚀能力
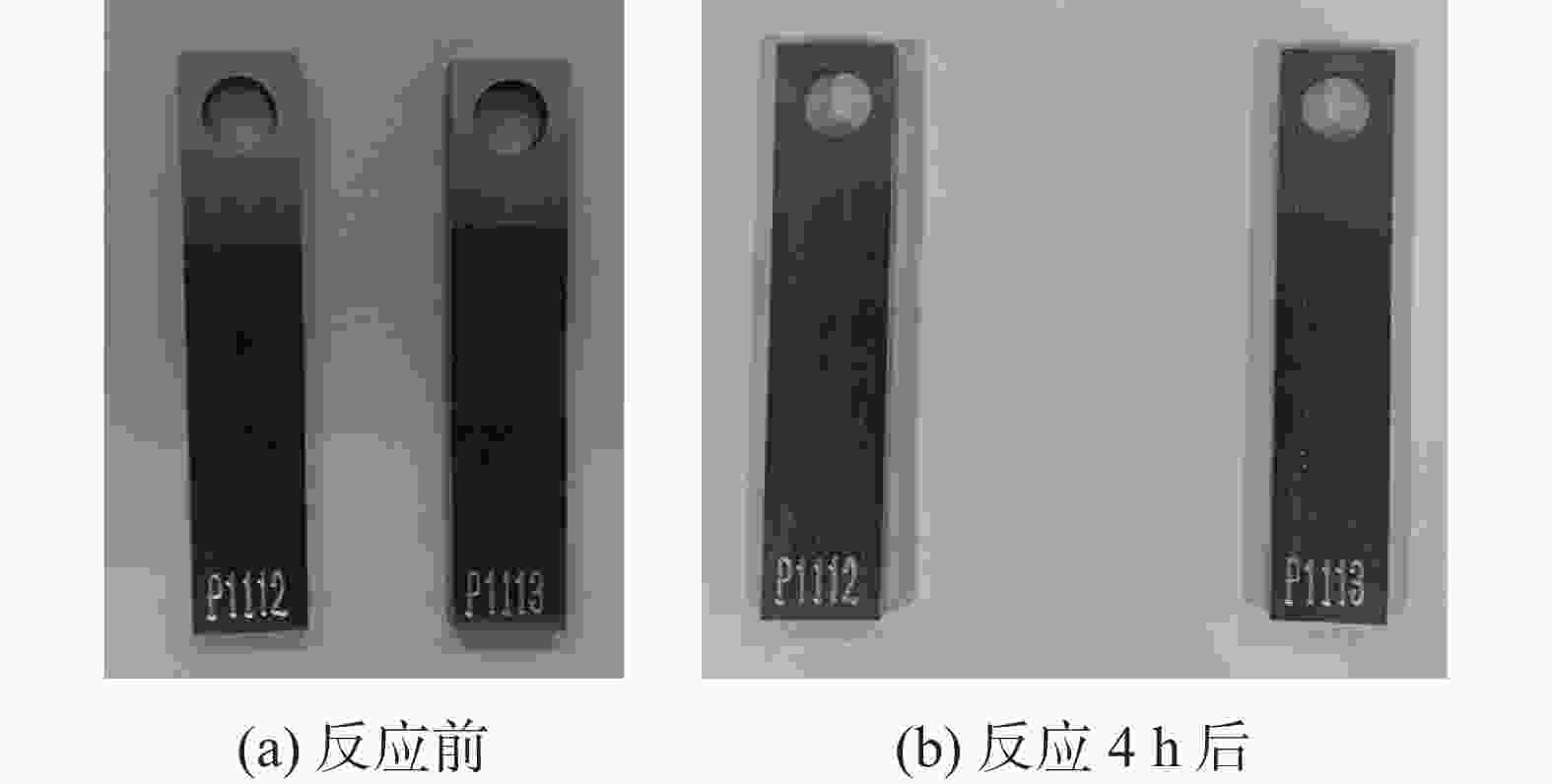
酸液体系 反应前质量/g 反应24 h后质量/g 溶解率/% 复合有机酸液 69.407 49.004 29.39 X 69.046 49.050 28.96 Y 69.236 50.569 26.96 Z 69.238 57.738 16.61 表 3 90 ℃下缓蚀剂SRAI-1对钢片的缓蚀效果
盐酸
浓度/%挂片
编号前重/
g后重/
g失重/
g腐蚀速率/
g·(m2·h)-1平均值/
g·(m2·h)-120.0 P1112 11.7733 11.7687 0.0046 0.85 0.78 P1113 11.7588 11.7550 0.0038 0.70 表 4 不同添加剂在酸液中的现象
加入的添加剂 室温 90 ℃ 有机酸A + 有机酸B 澄清、透明 澄清、透明 缓速剂Y 澄清、透明 澄清、透明 渗透剂W 澄清、透明 澄清、透明 降阻剂SRAP-2 澄清、透明 澄清、透明 缓蚀剂SRAI-1 澄清、透明 澄清、透明 铁离子稳定剂SRAF-1 澄清、透明 澄清、透明 加入所有添加剂 澄清、透明 澄清、透明 -
[1] HAN Dakuang, YANG Chengzhi, ZHANG Zhengqing, et al. Recent development of enhanced oil recovery in China[J]. Journal of Petroleum Science and Engineering, 1999, 22(1):181-188. [2] 卢志明,徐士鹏,艾尼·买买提,等. 海外碳酸盐岩油藏储层特征分析及其对产能影响[J]. 特种油气藏,2021,28(3):47-53.LU Zhiming, XU Shipeng, AINI Maimaiti, et al. Analysis of reservoir characteristics and their effect on productivity of overseas carbonate reservoirs[J]. Special Oil & Gas Reservoirs, 2021, 28(3):47-53. [3] XU Zhengxiao, LI Songyan, LI Binfei, et al. A review of development methods and EOR technologies for carbonate reservoirs[J]. Petroleum Science, 2020, 17(4):990-1013. doi: 10.1007/s12182-020-00467-5 [4] ZHAO Wenzhi, SHEN Anjiang, HU Suyun, et al. Geological conditions and distributional features of large-scale carbonate reservoirs onshore China[J]. Petroleum Exploration and Development, 2012, 39(1):1-14. doi: 10.1016/S1876-3804(12)60010-X [5] 王大鹏,陆红梅,陈小亮,等. 海相碳酸盐岩大中型油气田成藏体系及分布特征[J]. 石油与天然气地质,2016,37(3):363-371.WANG Dapeng, LU Hongmei, CHEN Xiaoliang, et al. Petroleum accumulation systems and distribution of medium to large marine carbonate fields[J]. Oil & Gas Geology, 2016, 37(3):363-371. [6] LI Yang, KANG Zhijiang, XUE Zhaojie, et al. Theories and practices of carbonate reservoirs development in China[J]. Petroleum Exploration and Development, 2018, 45(4):712-722. doi: 10.1016/S1876-3804(18)30074-0 [7] 吕海涛,韩俊,张继标,等. 塔里木盆地顺北地区超深碳酸盐岩断溶体发育特征与形成机制[J]. 石油实验地质,2021,43(1):14-22.LYU Haitao, HAN Jun, ZHANG Jibiao, et al. Development characteristics and formation mechanism of ultra-deep carbonate fault-dissolution body in Shunbei area, Tarim Basin[J]. Petroleum Geology & Experiment, 2021, 43(1):14-22. [8] 蒋廷学,周珺,贾文峰,等. 顺北油气田超深碳酸盐岩储层深穿透酸压技术[J]. 石油钻探技术,2019,47(3):140-147.JIANG Tingxue, ZHOU Jun, JIA Wenfeng, et al. Deep penetration acid-fracturing technology for ultra-deep carbonate oil & gas reservoirs in the Shunbei oil and gas field[J]. Petroleum Drilling Techniques, 2019, 47(3):140-147. [9] FAYZI P, MIRVAKILI A, RAHIMPOUR M R, et al. Experimental study of alcoholic retarded acid systems for high temperature gas wells acidizing process[J]. Chemical Engineering Research and Design, 2015, 93:576-583. doi: 10.1016/j.cherd.2014.06.003 [10] 陈赓良,黄瑛. 酸化工作液缓速作用的理论与实践[J]. 钻井液与完井液,2004(1):50-54,67. doi: 10.3969/j.issn.1001-5620.2004.01.015CHEN Gengliang, HUANG Ying. Retarded acting theory of acidizing fluid and its application[J]. Drilling Fluid & Completion Fluid, 2004(1):50-54,67. doi: 10.3969/j.issn.1001-5620.2004.01.015 [11] 姚远,袁志华. 适用于高温碳酸盐岩储层稠化缓速酸液体系研究[J]. 能源化工,2018,39(2):50-54. doi: 10.3969/j.issn.1006-7906.2018.02.011YAO Yuan, YUAN Zhihua. Study on the slow acid solution system for thickening of high temperature carbonate reservoir[J]. Energy Chemical Industry, 2018, 39(2):50-54. doi: 10.3969/j.issn.1006-7906.2018.02.011 [12] 李子甲,吴霞,黄文强. 深层碳酸盐岩储层有机酸深穿透酸压工艺[J]. 科学技术与工程,2020,20(20):8146-8151.LI Zijia, WU Xia, HUANG Wenqiang. Long penetration organic acid fracturing technique in deep carbonate reservoir[J]. Science Technology and Engineering, 2020, 20(20):8146-8151. [13] 王洋,袁清芸,吴霞,等. 耐温耐盐深穿透泡沫酸体系的研制及应用[J]. 油田化学,2018,35(3):406-410.WANG Yang, YUAN Qingyun, WU Xia. Preparation and application of deep penetration foam acid system with higher heat resistance and salt tolerance[J]. Oilfield Chemistry, 2018, 35(3):406-410. [14] REYES E, BEUTERBAUGH A, ASHCRAFT P, et al. Retarding HCl acid reactivity without gelling agents, emulsifiers, or polymers for low to high temperature acidizing applications [C]// SPE International Conference and Exhibition on Formation Damage Control, Lafayette: OnePetro, 2020: SPE-199284-MS. [15] 穆代峰,贾文峰,姚奕明,等. 胶凝酸与交联酸一体化耐高温缓速酸研究[J]. 钻井液与完井液,2019,36(5):634-638. doi: 10.3969/j.issn.1001-5620.2019.05.019MU Daifeng, JIA Wenfeng, YAO Yiming, et al. Study on the integration of gelled acid and crosslinked acid to form high temperature retarded acid[J]. Drilling Fluid & Completion Fluid, 2019, 36(5):634-638. doi: 10.3969/j.issn.1001-5620.2019.05.019 [16] 何州,郭涛. 缓速酸酸液缓速性能研究[J]. 化工设计通讯,2019,45(12):147+150.HE Zhou, GUO Tao. Research on retarding performance of retarding acid and acid solution[J]. Chemical Engineering Design Communications, 2019, 45(12):147+150. [17] ALKHALDI M H, NASR-EL-DIN H A, SARMA H. Kinetics of the reaction of citric acid with calcite[J]. Chemical Engineering Science, 2007, 62(21):5880-5896. doi: 10.1016/j.ces.2007.06.021 [18] SHEN Xin, WANG Shibin, GUO Jianchun, et al. Effect of carbon chain lengths of cationic surfactant on inhibition rate of acid-rock reaction[J]. Journal of Petroleum Science and Engineering, 2021, 196:107793. doi: 10.1016/j.petrol.2020.107793 [19] COOPER T G, DE LEEUW N H. A computer modeling study of the competitive adsorption of water and organic surfactants at surfaces of the mineral scheelite[J]. Langmuir, 2004, 20(10):3984-3994. doi: 10.1021/la049796w [20] GAO Zhiyong, SUN Wei, HU Yuehua. New insights into the dodecylamine adsorption on scheelite and calcite: An adsorption model[J]. Minerals Engineering, 2015, 79:54-61. doi: 10.1016/j.mineng.2015.05.011 [21] FREEMAN C L, ASTERIADIS I, YANG M, et al. Interactions of organic molecules with calcite and magnesite surfaces[J]. The Journal of Physical Chemistry C, 2009, 113(9):3666-3673. doi: 10.1021/jp807051u [22] 张春雨,吴家全,王桂珠,等. 胍胶降阻特性及机理研究[J]. 应用化工,2020,49(1):63-66.ZHANG Chunyu, WU Jiaquan, WANG Guizhu, et al. Study on the drag reduction and mechanism of guanidine gum[J]. Applied Chemical Industry, 2020, 49(1):63-66. [23] 龚迪光,曲占庆,郭天魁,等. 径向井水力压裂摩阻影响因素与计算公式[J]. 钻井液与完井液,2016,33(3):102-106. doi: 10.3969/j.issn.1001-5620.2016.03.021GONG Diguan, QU Zhanqing, GUO Tiankui, et al. Factors affecting friction loss of hydraulic fracturing in ultra-short radius radial wells and the calculating equation thereof[J]. Drilling Fluid & Completion Fluid, 2016, 33(3):102-106. doi: 10.3969/j.issn.1001-5620.2016.03.021 [24] 钟燕清,张永清,陈宪方,等. 不同螯合剂对零价铁活化过硫酸盐降解对氯苯胺的影响[J]. 环境化学,2015,4:685-691. doi: 10.7524/j.issn.0254-6108.2015.04.2014101302ZHONG Yanqing, ZHANG Yongqing, CHEN Xianfang, et al. Effect of chelating agents on the degradation of p-chloroaniline in Fe0-persulfate system[J]. Environmental Chemistry, 2015, 4:685-691. doi: 10.7524/j.issn.0254-6108.2015.04.2014101302 [25] 王旭,尹玉川. 表面活性剂缓速酸酸压实践[J]. 钻采工艺,2004,27(5):40-44.WANG Xu,YIN Yuchuan. Acid Fracturing Practice of Surfactant Retarded Acid[J]. Drilling & Production Technology, 2004, 27(5):40-44. [26] 王萌,车明光,周长林,等. 一种新型耐高温碳酸盐岩酸压胶凝酸及其应用[J]. 钻井液与完井液,2020,37(5):670-676.WANG Meng, CHE Mingguang, ZHOU Changlin,et al. A novel gelled acid for the acid fracturing of the high-temperature carbonates and its application[J]. Drilling Fluid & Completion Fluid, 2020, 37(5):670-676. -




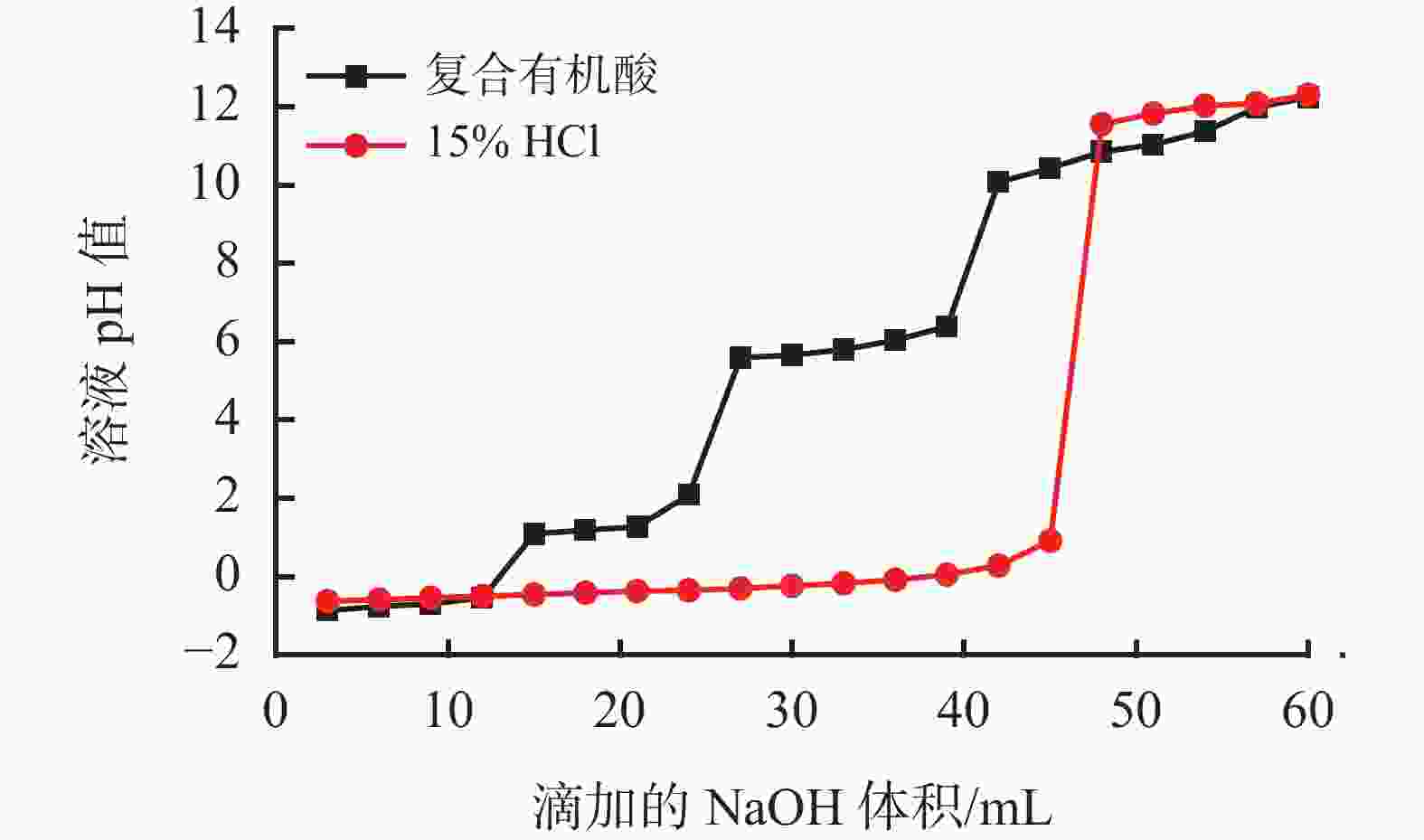
 下载:
下载: