Effect of Water on BaSO4 Particles/GTL Suspensions
-
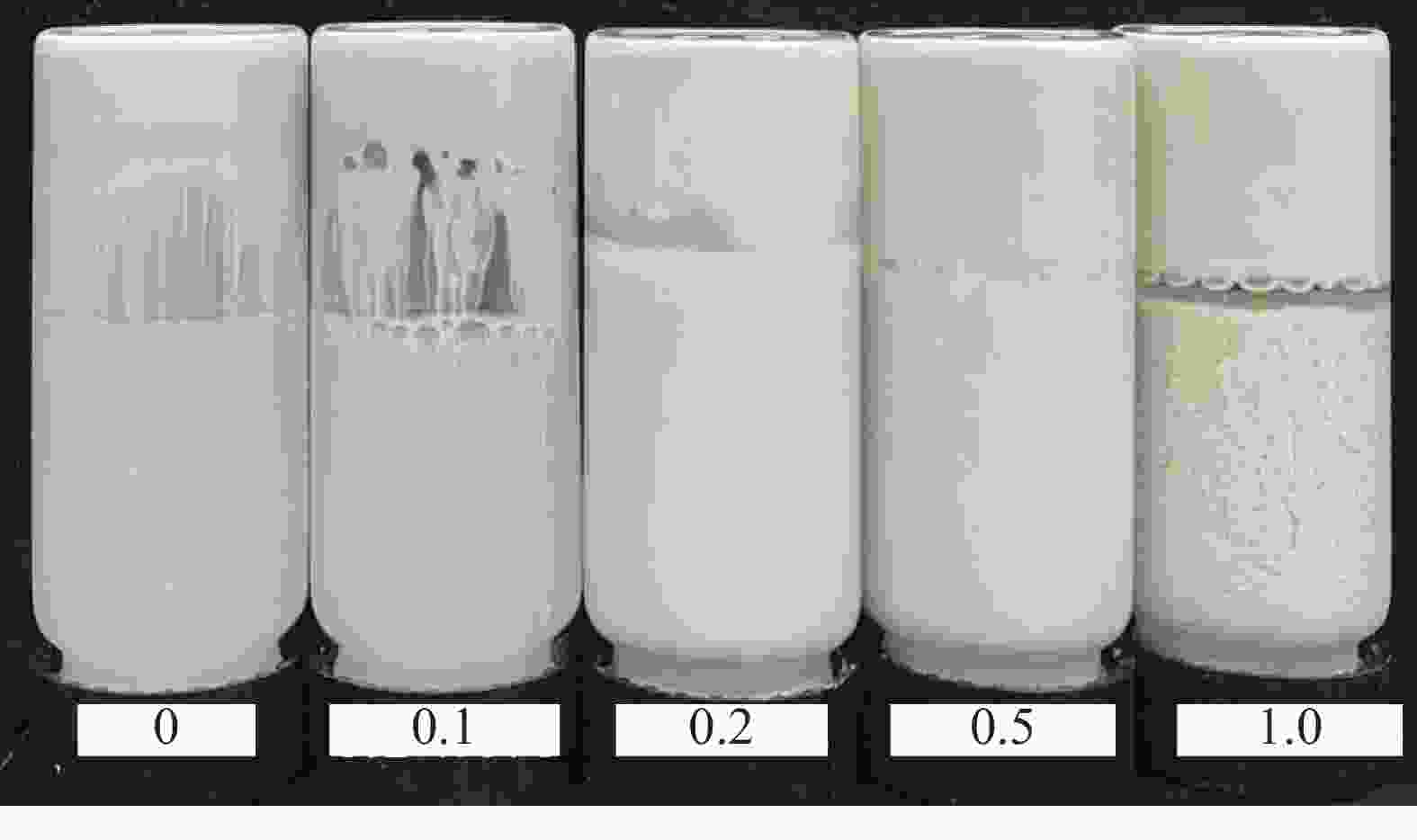
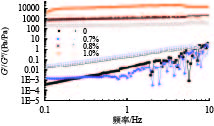
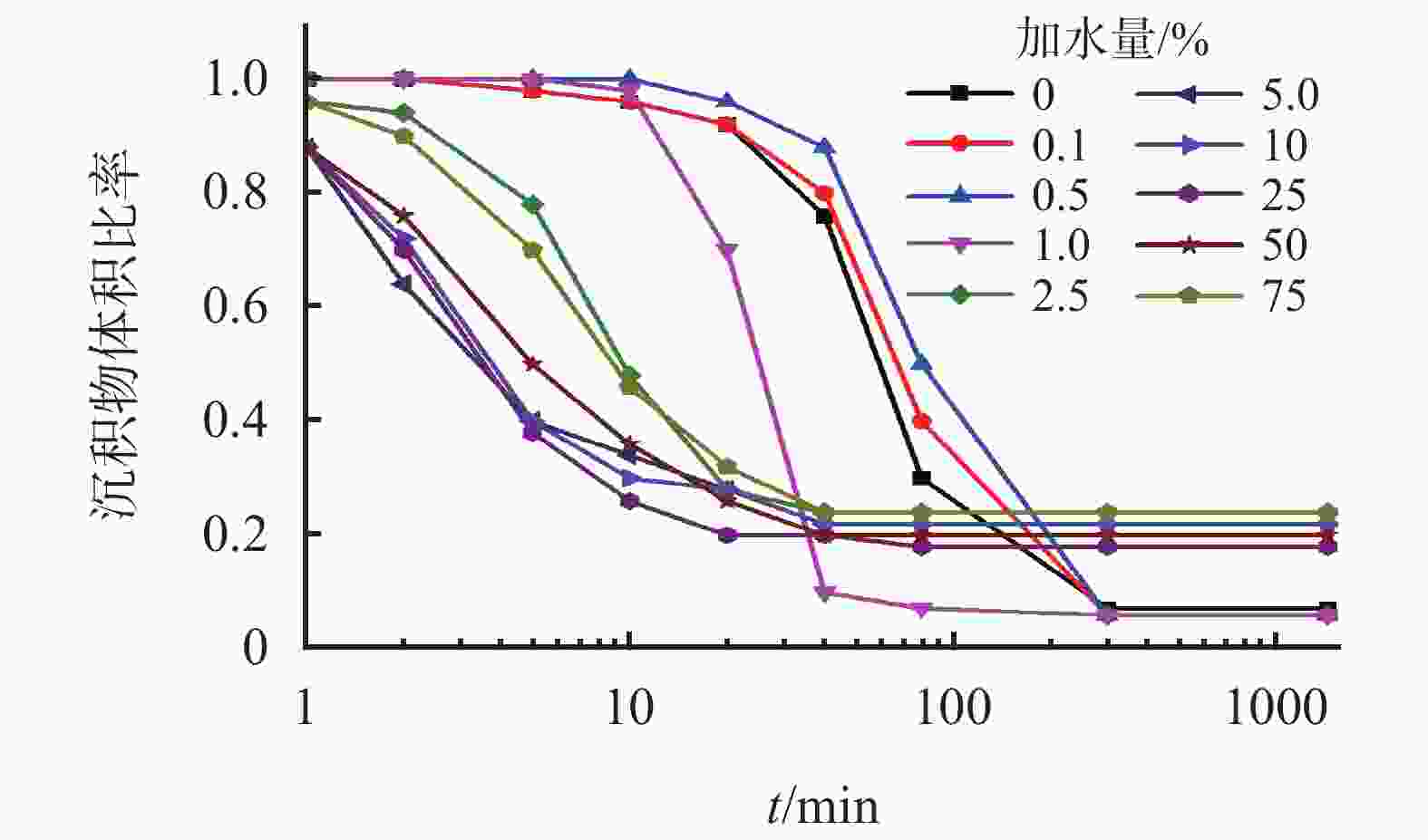
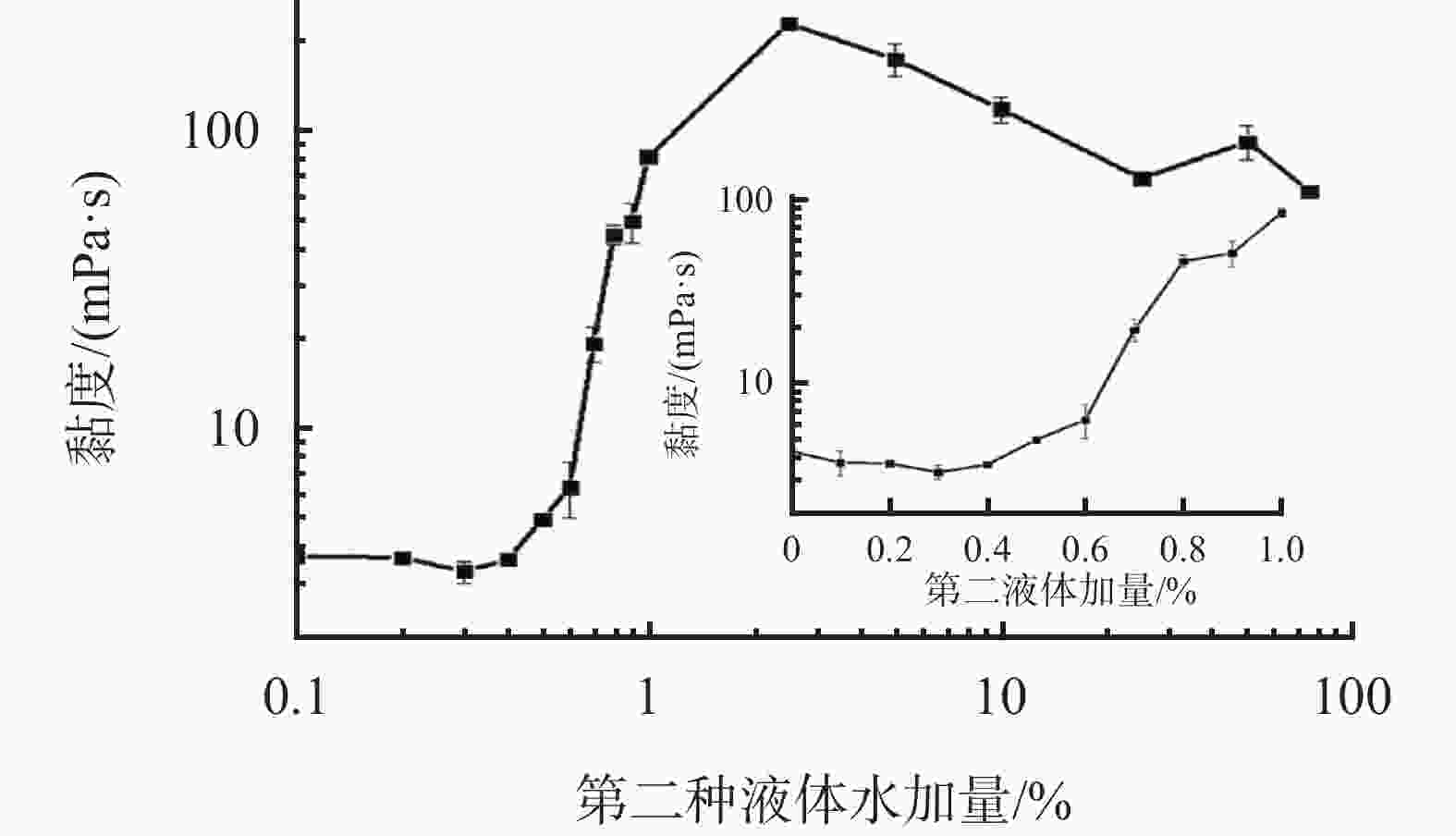
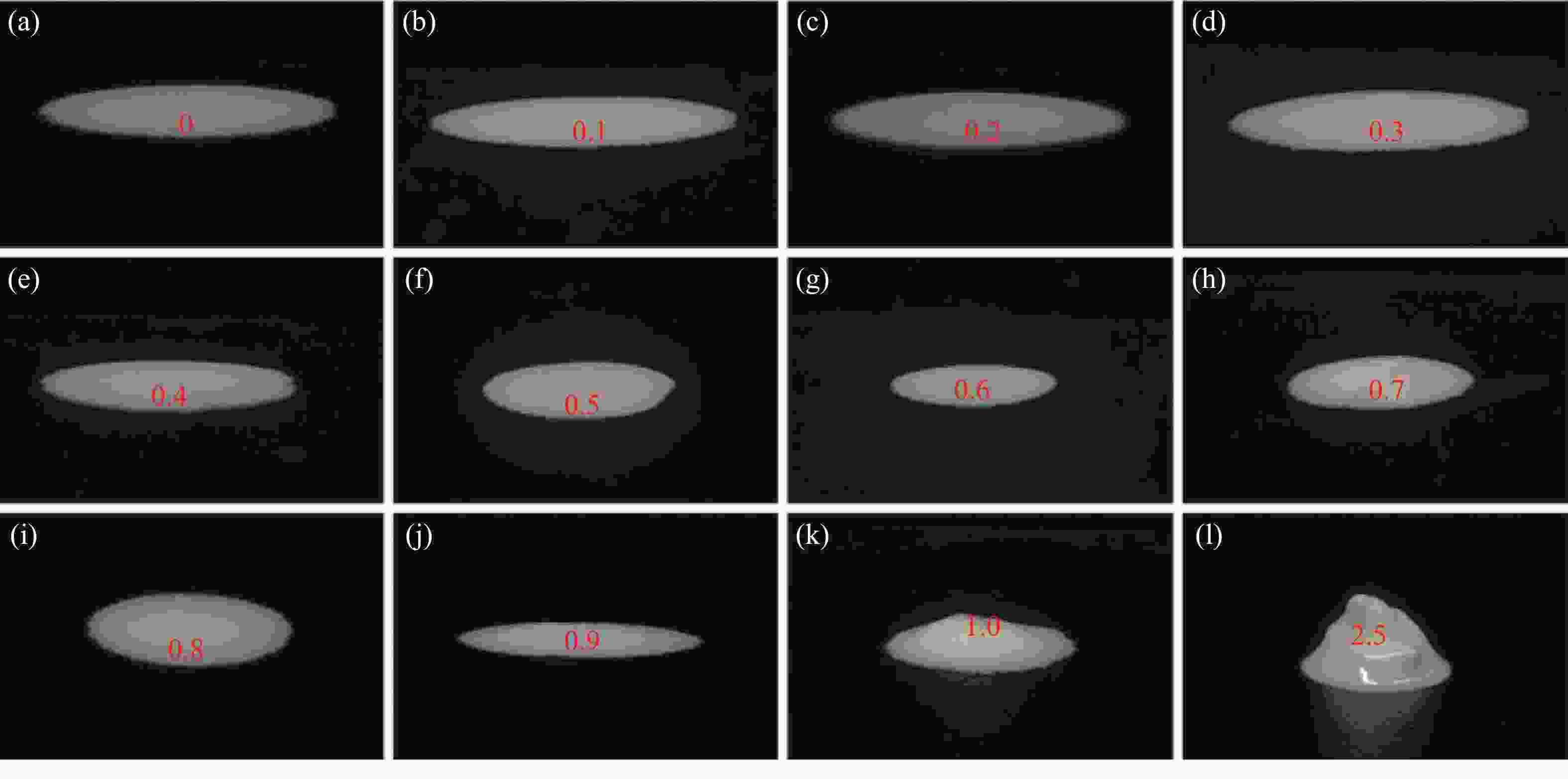
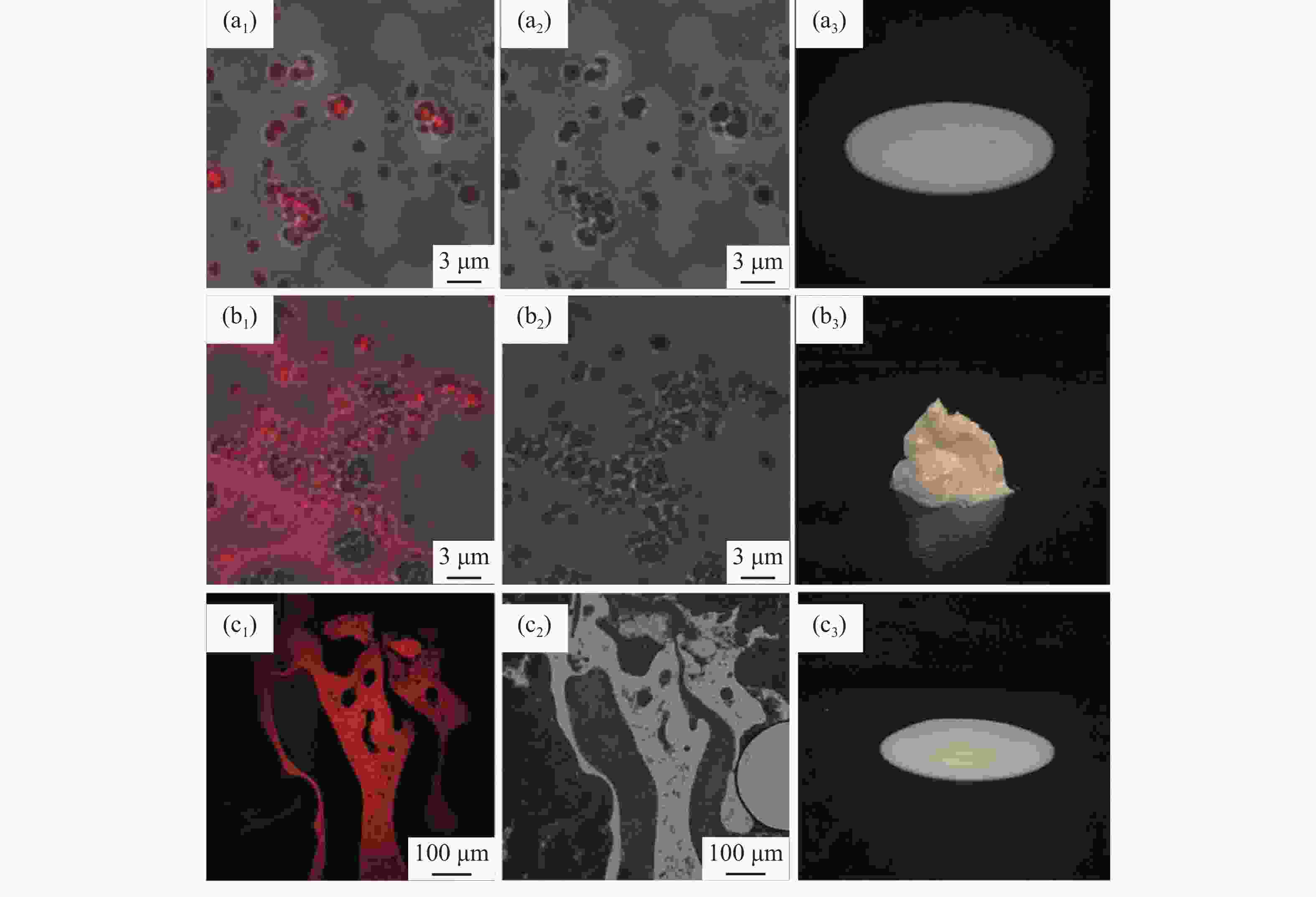
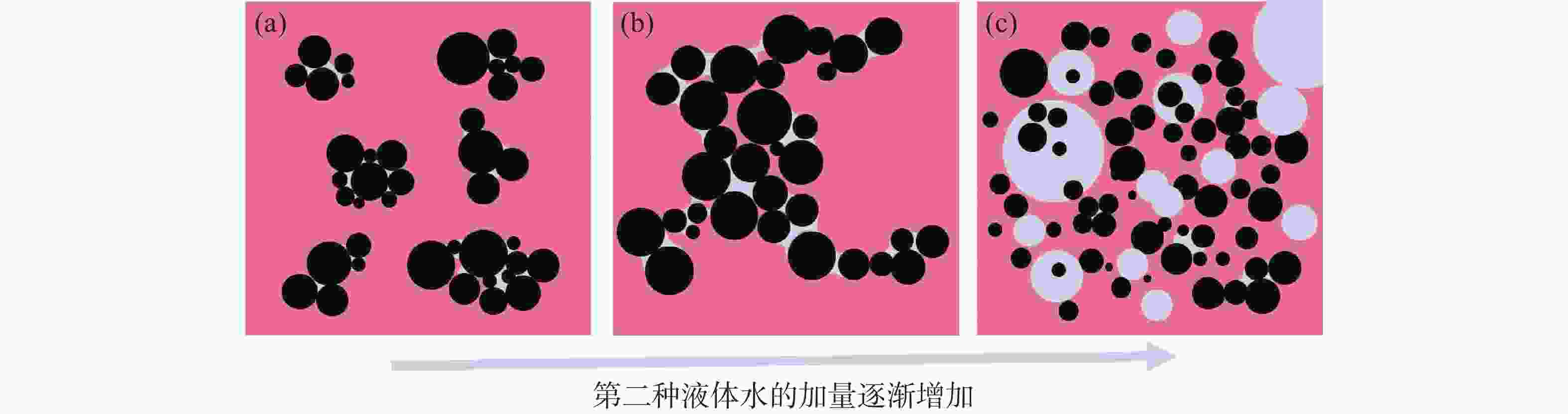
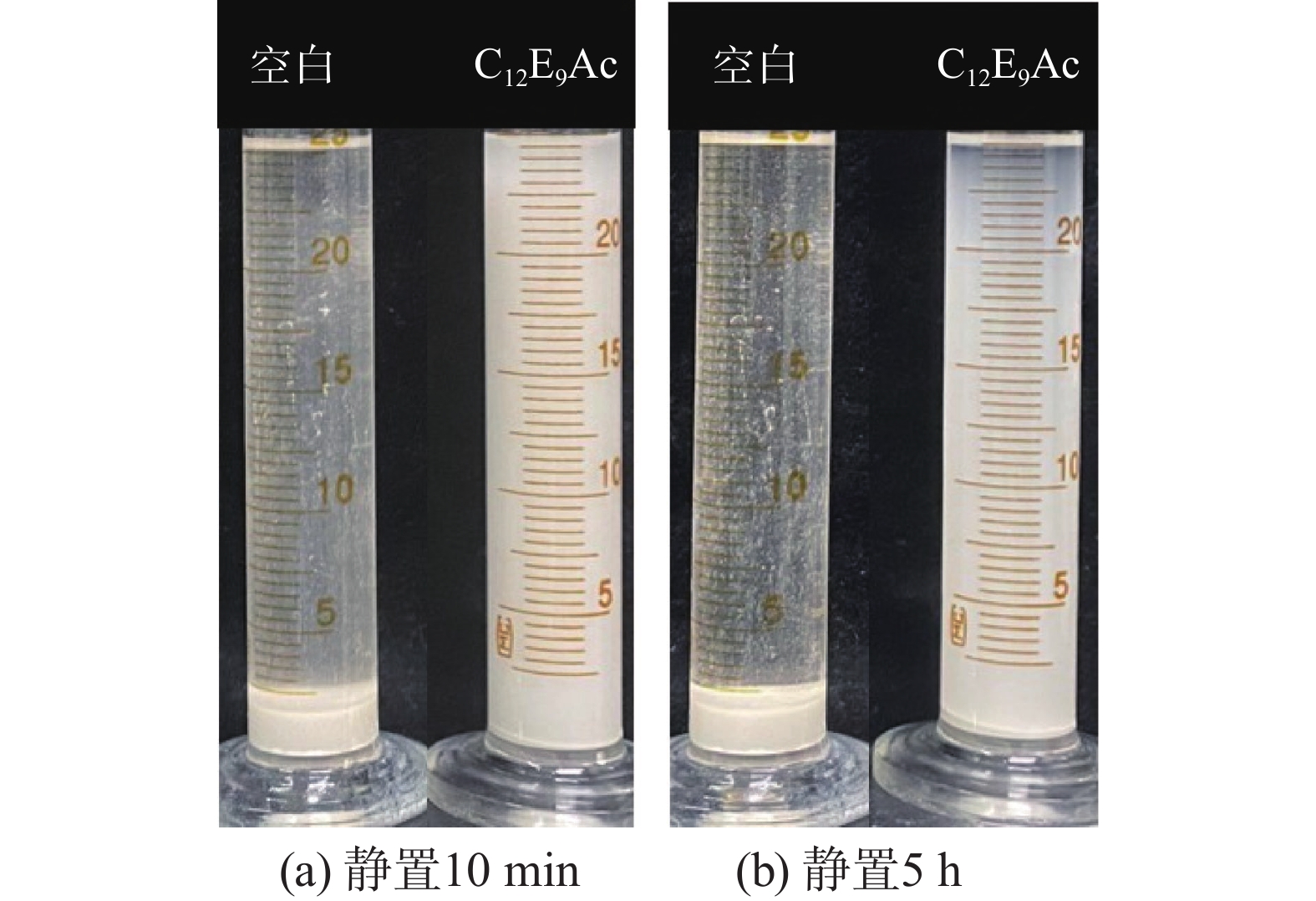
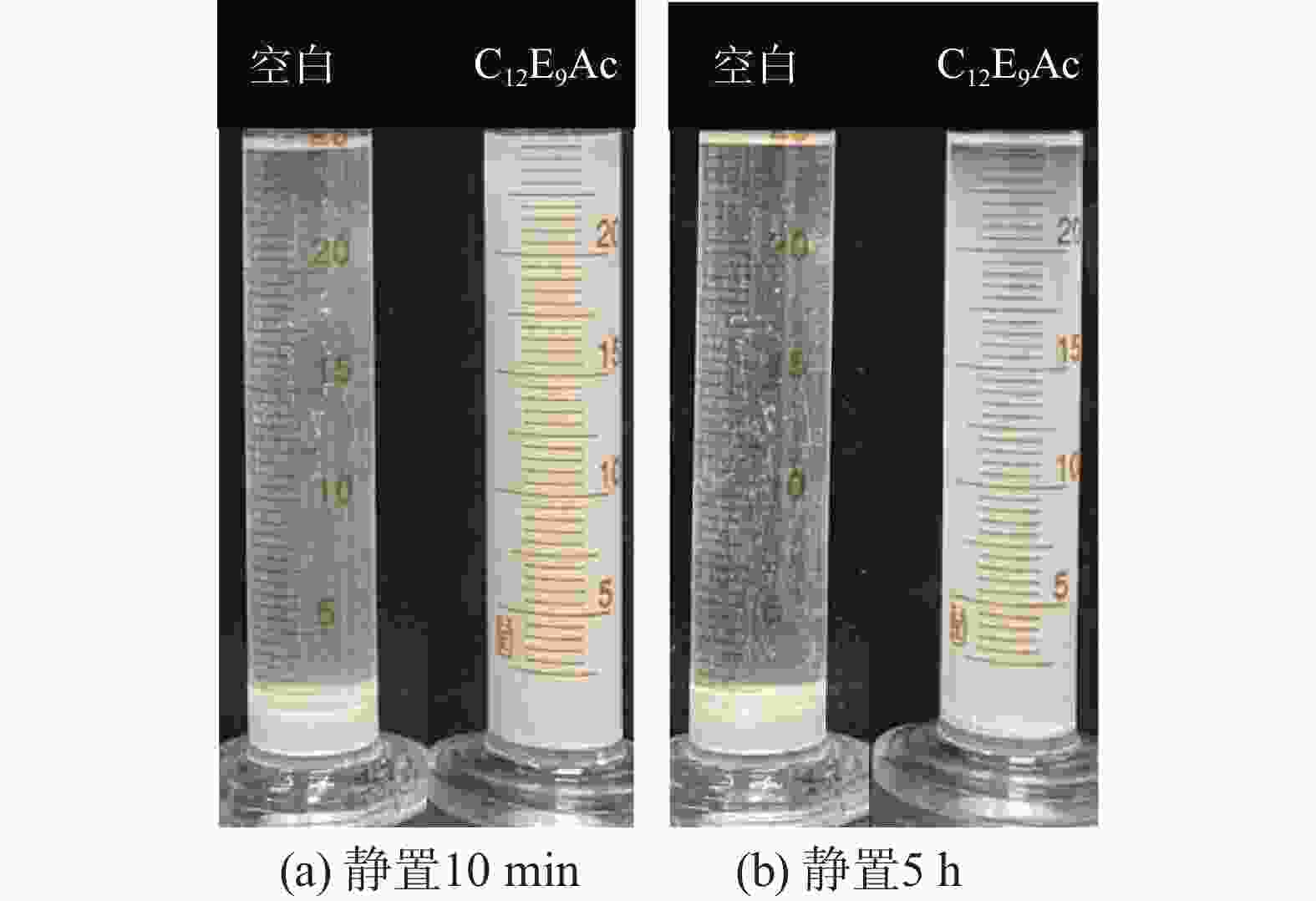
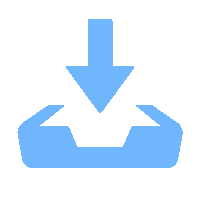
摘要: 针对加重材料的分散状态对油基钻井液的流变性能有较大影响,以脂肪醇聚氧乙烯醚羧酸(C12E9Ac)为分散剂,BaSO4颗粒为分散相,气制油为分散介质,探讨了水对悬浮体系中颗粒沉降稳定性和体系黏度的影响。结果表明,随着水加量的增加,可以使分散颗粒间由以排斥力为主导转化为以吸引力为主导,当水加量小于0.5%(占BaSO4颗粒质量的百分数)时,悬浮液体系的沉降稳定性和黏度几乎不受影响,且再分散性提高;当加量为0.5%~2.5%时,水不仅可以使悬浮液体系中颗粒发生聚集,并且聚集形成的“大颗粒”也在水桥作用下连接在一起,形成复杂的网络结构;当加量大于25%时,悬浮液体系中部分颗粒已处于水相中,使颗粒的网络结构减少,导致悬浮液的黏度降低。因此,向C12E9Ac稳定的悬浮液中引入适量水,能够实现在分散稳定性和黏度几乎不发生变化的前提下,提高沉积物的再分散性,为调控油基钻井液中颗粒沉降稳定性提供了研究方法。Abstract: The dispersion state of weight material has a great effect on the rheology of oil based drilling fluids. In laboratory experiment, the effects of water on the settling stability and the viscosity of the suspension were studied. The suspension used in the experiments was formulated with fatty alcohol polyoxyethylene ether carboxylic acid (C12E9Ac) as the dispersant, BaSO4 as the dispersed phase and a gas to liquids (GTL) as the dispersing medium. It was found through the experiments that with the increase in the water content in the suspension, the dominant forces between the dispersed particles can be turned from repulsive force to attractive force. When the water is added in a concentration that is less than 0.5%, the settling stability and viscosity of the suspension are almost not affected, and the re-dispersibility of the suspension is enhanced. When the water is added at a concentration between 0.5% and 2.5%, water will not only cause the particles in the suspension to aggregate, the “big particles” formed by the aggregation of the particles will connect with each other through water bridge to form a complex network structure. When the water is added at a concentration greater than 25%, part of the particles in the suspension is dispersed in the water phase, decreasing the network structures formed by the particles, and hence the viscosity of the suspension. Thus, adding some water into a suspension stabilized by the C12E9Ac will help improve the re-dispersibility of the sediments in the suspension without disturbing the dispersion stability and viscosity of the suspension. The conclusions drawn from these experiments have provided a research method for controlling the settling stability of the particles in an oil based drilling fluid.
-
-
[1] 潘谊党,于培志. 密度对油基钻井液性能的影响[J]. 钻井液与完井液,2019,36(3):273-279. doi: 10.3969/j.issn.1001-5620.2019.03.002PAN Yidang, YU Peizhi. Effect of density on the performance of oil base drilling fluids[J]. Drilling Fluid & Completion Fluid, 2019, 36(3):273-279. doi: 10.3969/j.issn.1001-5620.2019.03.002 [2] 李炎军,胡友林,吴江,等. 油基钻井液润湿剂评价新方法[J]. 钻井液与完井液,2019,36(1):46-50. doi: 10.3969/j.issn.1001-5620.2019.01.009LI Yanjun, HU Youlin, WU Jiang, et al. A new method for evaluating wetting agents used in oil base drilling fluid[J]. Drilling Fluid & Completion Fluid, 2019, 36(1):46-50. doi: 10.3969/j.issn.1001-5620.2019.01.009 [3] ZHANG J, ZHAO H, LI W, et al. Multiple effects of the second fluid on suspension viscosity[J]. Scientific reports, 2015, 5(1):1-8. doi: 10.9734/JSRR/2015/14076 [4] DE VRIES A, JANSEN D, VANDER LINDEN E, et al. Tuning the rheological properties of protein-based oleogels by water addition and heat treatment[J]. Food Hydrocolloids, 2018(79):100-109. [5] HOFFMANN S, KOOS E, WILLENBACHER N. Using capillary bridges to tune stability and flow behavior of food suspensions[J]. Food Hydrocolloids, 2014(40):44-52. [6] WEN W J, HUANG X X, YANG S H, et al. The giant electrorheological effect in suspensions of nanoparticles[J]. Nature Materials, 2003, 2(11):727-730. doi: 10.1038/nmat993 [7] RIGDEN P J. Rheology of suspensions of high solid concentration[J]. Nature, 1951, 167(4240):197-198. doi: 10.1038/167197a0 [8] CHATTERJI A K, KAPSE G W. Rheology of dilute aqueous suspensions of some reactive solids[J]. Nature, 1963, 200(490):868-869. [9] KOOPAL L K. Wetting of solid surfaces: fundamentals and charge effects[J]. Advances in Colloid and Interface Science, 2012(179):29-42. [10] SOLOMON M J, SAEKI T, WAN M, et al. Effect of adsorbed surfactants on the rheology of colloidal zirconia suspensions[J]. Langmuir, 1999, 15(1):20-26. doi: 10.1021/la9706577 [11] STICKEL J J, POWELL R L. Fluid mechanics and rheology of dense suspensions[J]. Annual Review of Fluid Mechanics, 2005(37):129-149. [12] KOOS E, WILLENBACHER N. Capillary forces in suspension rheology[J]. Science, 2011(331):897-900. [13] KOOS E, WILLENBACHER N. Particle configurations and gelation in capillary suspensions[J]. Soft Matter, 2012, 8(14):3988-3994. doi: 10.1039/c2sm07347a [14] KOOS E, JOHANNSMEIER J, SCHWEBLER L, et al. Tuning suspension rheology using capillary forces[J]. Soft Matter, 2012, 8(24):6620-6628. doi: 10.1039/c2sm25681a [15] LENNART B, EVA S. Temperature Induced Gelation of Concentrated Ceramic Suspensions: Rheological Properties[J]. Journal of the European Ceramic Society, 1999, 19(12):2117-2123. doi: 10.1016/S0955-2219(99)00021-7 [16] DANOV K D, GEORGIEV M T, KRALCHEVSKY P A, et al. Hardening of particle/oil/water suspensions due to capillary bridges: Experimental yield stress and theoretical interpretation[J]. Advances in Colloid and Interface Science, 2018(51):80-96. -




 下载:
下载: